Poly (ADP-ribose) polymerase (PARP), a family of proteins, is involved in various cellular processes such as DNA repair and programmed cell death. PARP is present in the cell nucleus. It consists of a DNA-binding domain, a caspase-cleaved domain, an auto-modification domain, and a catalytic domain. In particular, the protein can detect and initiate an immediate cellular response to metabolic, chemical, or radiation-induced single-strand DNA breaks (SSB). Once PARP detects SSB, it will bind to the DNA, and begins to synthesize a signal for DNA-repairing enzymes. PARP plays an important role in cell proliferation, thus, PARP inhibitors have been used for researching anticancer.
Simmiparib is a highly potent and orally active PARP1 and PARP2 inhibitor.
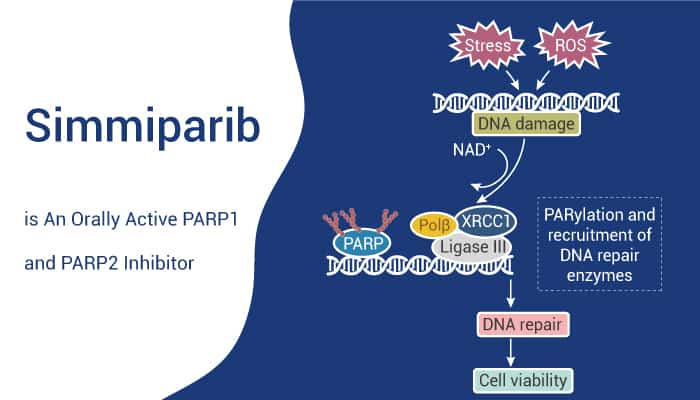
Simmiparib exhibits inhibitory activity against PARP1 and PARP2 with IC50 values of 1.75 nM and 0.22 nM, respectively. It has more potent PARP1/2 inhibition than its parent Olaparib. Simmiparib can increase the phosphorylation levels of Chk1 and Chk2; as well as improve the protein levels of p-Cyclin B1 (S147), Cyclin B1, p-CDK1 (Y15), and CDK1 in Capan-1 cells. Furthermore, this agent induces DNA double-strand breaks accumulation and G2/M arrest in homologous recombination repair (HR)-deficient cells, thereby inducing apoptosis.
Researches show that Simmiparib exhibits remarkable anticancer activities in cells and nude mice bearing xenografts. The PARP inhibitor can inhibit various mutant cancer cells at the nanomolar scale, such as BRCA1– MDA-MB-436 (IC50 = 0.2 nM). Moreover, Simmiparib (p.o.; 14 days) inhibits the growth of tumors in BRCA2-/- V-C8 and BRCA2-/- MDA-MB-436 xenograft mice models. It exhibits average inhibition rates of 64.93%, 82.98%, and 85.79% at 2, 4, and 8 mg/kg in mice. Besides, Simmiparib suppresses dose-dependently the growth of BRCA1-mutated breast cancer in xenograft mice model; exhibiting the inhibition rate of 76.73% and 93.82% at 10 mg/kg and 50 mg/kg, respectively.
In conclusion, Simmiparib is an excellent PARP1 and PARP2 inhibitor, possessing anticancer effects.
References:
Yuan B, et al. Cancer Lett. 2017 Feb 1;386:47-56.