Integrin β7 is an integrin protein that in humans is encoded by the ITGB7 gene. Besides, It can pair with ITGA4 (CD49d) to form the heterodimeric integrin receptor α4β7, or with ITGAE (CD103) to form αEβ7. α4β7 integrin plays a crucial role in leucocyte homing to the intestinal mucosa and associated lymphoid tissues. While, The αEβ7 integrin on leucocytes is thought to mediate the retention of cells, particularly IELs, in mucosal tissue. Additionally, Anti‐αEβ7 antibody treatment shows to attenuate the severity of colitis in a mouse experimental colitis model. Moreover, studies show that the dual blockade of αEβ7 and α4β7 integrins reduced immune cell infiltration in the colon compared with the blockade of anti‐α4β7 alone. So, Here we will introduce an anti-β7 integrin monoclonal antibody.
Etrolizumab (rhuMAb Beta7) is a gut-selective, anti-β7 integrin monoclonal antibody.
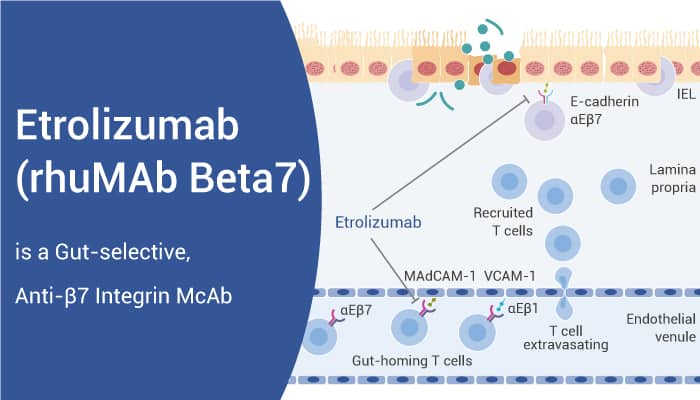
Etrolizumab is specifically targeting the β7 subunit of α4β7 and αEβ7 integrins with Ki values of 18 nM and 1800 pM for Human α4β7 and Human αEβ7-293, respectively. In addition, Etrolizumab (RPMI 8866 cells and αEβ7-293 cells) blocks the interaction of α4β7 with its cognate ligands MAdCAM-1 and VCAM-1 with IC50 values of 0.075 and 0.089 nM, respectively. Moreover, It also blocks the interaction between αEβ7 and its ligand E-cadherin with an IC50 value of 3.96 nM.
Etrolizumab has the potential for the research of inflammatory bowel disease (IBD). Specifically, Etrolizumab (5 mg/kg; i.v.; once) decreases β7 integrins on T lymphocytes in normal female BALB/c mice. Which shows 98.3% of intraepithelial CD8+ T-cell β7 integrin receptors and 90.0% of intraepithelial CD4+ T-cell β7 integrin receptors after 24 h. Nonetheless, Etrolizumab (200 µg (100 µL); i.p.; once) inhibits lymphocyte homing in the CD45RBhigh T cell-reconstituted SCID mouse model of colitis.
All in all, Etrolizumab is a gut-selective, anti-β7 integrin monoclonal antibody. Besides, Etrolizumab has the potential for the research of inflammatory bowel disease (IBD).
Reference:
[1] Tang MT, et al. Aliment Pharmacol Ther. 2018 Jun;47(11):1440-1452.
[2] Stefanich EG, et al. Br J Pharmacol. 2011 Apr;162(8):1855-70.