Interleukin-3 receptor (CD123) is a soluble cytokine that is important for the immune system. CD123 is the receptor of interleukin 3 α Chain is a cytokine receptor. Meanwhile, CD123 is overexpressed in a variety of hematological malignancies. Compared with non-tumor hematopoietic stem cells, CD123 expression was high in leukemia stem cells. This makes it a useful biomarker for the diagnosis and treatment of hematological malignancies.
Nonetheless, monoclonal antibodies are biological drugs for targeting acute myeloid leukemia in vivo. Besides, CD123 is a surface marker overexpressed in a variety of hematological diseases (including acute myeloid leukemia). Efforts to target CD123 have led to the development of drugs that can effectively target tumor cells expressing this molecule. Let’s study an IgG1 CD123-neutralizing monoclonal antibody, Talacotuzumab.
Talacotuzumab is an IgG1 CD123-Neutralizing Monoclonal Antibody.
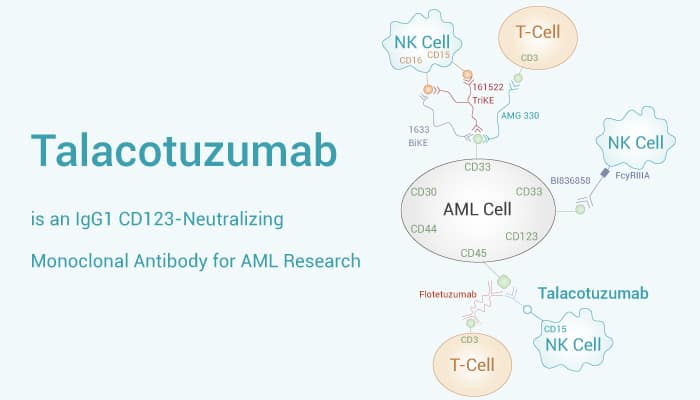
Above all, Talacotuzumab (JNJ 56022473; CSL 362) is an IgG1-type fully humanized, CD123-neutralizing monoclonal antibody containing a modified Fc structure. Moreover, Talacotuzumab has KDs of 0.43 nM, 188 nM, 46 nM, 16.8 nM for CD123, CD32b/c, CD16-158F, and CD16-158V, respectively. Furthermore, Talacotuzumab inhibits IL-3 binding to CD123, antagonizing IL-3 signaling in target cells. Talacotuzumab has mutated the Fc region to increase affinity for CD16 (FcγRIIIa), thereby enhancing antibody-dependent cell-mediated cytotoxicity (ADCC). Importantly, Talacotuzumab is highly effective in vivo in reducing leukemic cell growth in acute myeloid leukemia (AML) xenograft mouse models.
Next in importance, Talacotuzumab strongly mediates ADCC of TF-1 cells with an ic50 of 5 ng/ml (33 pM). Particularly, Talacotuzumab inhibited TLR7-stimulated and TLR9-stimulated IFN-α production in both SLE donors and healthy donors plasmacytoid dendritic cells (pDCs) and basophils. Obviously, Talacotuzumab inhibits TLR7- and TLR9-induced plasmablast expansion and proliferation by depletion of plasmacytoid dendritic cells (pDCs).
Once again, Talacotuzumab results in a significant delay in tumor growth compared with isotype control in acute myeloid leukemia mice xenografts. Additionally, Talacotuzumab has maximal serum concentrations at 48 hours of ~12, 190, and 380 μg/ml at doses of 1, 10, and 30 mg/kg in naive cynomolgus monkeys, respectively. Interestingly, Talacotuzumab for 3 weeks significantly reduced engraftment of AML-5, -16, and -17 in the blood, spleen, and liver. But it was only significantly effective against AML-17 in the bone marrow in Female OD/SCID mice.
All in all, Talacotuzumab is an IgG1-type CD123-neutralizing monoclonal antibody.
References:
S J Busfield, et al. Leukemia. 2014 Nov;28(11):2213-21.
Shereen Oon, et al. JCI Insight. 2016 May 5;1(6):e86131.