HDAC (Histone deacetylases) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on a histone, allowing the histones to wrap the DNA more tightly. Firstly, this is important because DNA is wrapped around histones and DNA expression is regulated by acetylation and de-acetylation. Also, its action is opposite to that of histone acetyltransferase. Secondly, HDAC proteins are now also called lysine deacetylases (KDAC), to describe their function rather than their target. Importantly, it also includes non-histone proteins. Together with the acetylpolyamine amidohydrolases and the acetoin utilization proteins, the histone deacetylases form an ancient protein superfamily known as the histone deacetylase superfamily.
Romidepsin (FK 228) is an HDAC inhibitor for cancer research.
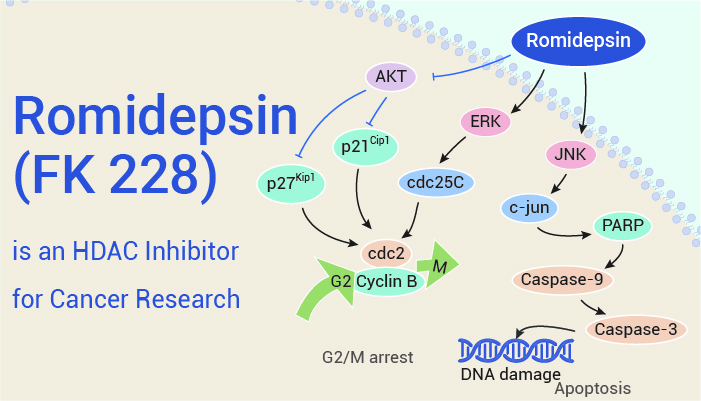
Romidepsin (FK 228) is a HDAC inhibitor with anti-tumor activities. Importantly, it inhibits HDAC1, HDAC2, HDAC4, and HDAC6 with IC50s of 36 nM, 47 nM, 510 nM, and 1.4 μM, respectively. Besides, Romidepsin is produced by Chromobacterium violaceum. Also, it induces cell G2/M phase arrest and apoptosis. Firstly, Romidepsin (0-72 h; 0 -80 nM) inhibits HCC cell proliferation in a dose-dependent manner. Secondly, it (0-48 h; 0-60 nM) causes a time- and dose-dependent induction of cell cycle arrest in the G2/M phase in HCC cells. Moreover, Romidepsin (0-48 hours; 0-60 nM) promotes apoptosis and increases c-caspase-3, c-caspase-9, and c-PARP protein expression in HCC cells. In in vivo studies, Romidepsin (i.p.; 0.5 and 1 mg/kg; every 3 days; 21 days) inhibited tumor growth, showing high expression of p-cdc25C, ki67, c-caspase-3, and c-PARP, and Reduced expression of Ki-67 in tumors.
In conclusion, Romidepsin (FK 228), an HDAC inhibitor with antineoplastic activity, induces G2/M cell cycle arrest and apoptosis.
References:
[1] Ryohei Furumai, et al. Cancer Res. 2002 Sep 1;62(17):4916-21.
[2] Wei-Jian Sun, et al. Biochem Pharmacol. 2017 Mar 1;127:90-100.