v-Raf murine sarcoma viral oncogene homolog B (BRAF) is the part of the mitogen-activated protein kinase (MAPK) signaling pathway. BRAF was first discovered in 2002. The discovery of BRAF mutations ushered in a new era of targeted therapy for patients with malignant melanoma, colorectal cancer, and non-small cell lung cancer. In the past, vemurafenib is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of BRAF V600-mutated melanomas. Today, we will introduce the first selective BRAF inhibitor –Vemurafenib.
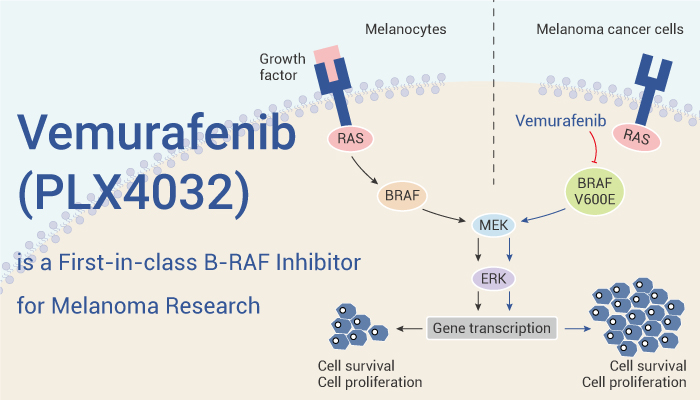
Vemurafenib (PLX4032) is a first-in-class, selective, potent inhibitor of B-RAF kinase for melanoma research.
Vemurafenib (PLX4032) is a B-RAF kinase inhibitor with IC50s of 31 and 48 nM for RAF V600E and c-RAF-1, respectively. First, Vemurafenib selectively blocks the RAF/MEK/ERK pathway in BRAF mutant cells. Second, It induces MEK and ERK phosphorylation at high concentrations in CHL-1 cells. In vivo, Vemurafenib ( 20, 25, 75 mg/kg, p.o.) causes dose-dependent inhibition of tumor growth, with higher exposures resulting in tumor regression of BRAF mutant xenografts.
Reference:
[1] Bollag G, et al. Nature, 2010, 467(7315), 596-599.
[2] Yang H, et al. Cancer Res, 2010, 70(13), 5518-5527.
[3] Wanchoo R, et al. Clin Kidney J. 2016 Apr;9(2):245-51.