T cells are important immune cells in the human body and express co-stimulating immune checkpoint proteins on their surface. Through immune checkpoints, cancer cells can block the activation of T cells and their cytotoxic effects on tumors, leading to immune evasion. As surface receptor proteins, immune checkpoints can be effectively inhibited by antibodies known as checkpoint inhibitors.
Anti-programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies are the most successful checkpoint inhibitor in clinical application. On the one hand, antibody blockade of PD-1 prevents its interaction with PD-L1 and PD-L2. On the other hand, it blocks their downstream pathways and recovering the anti-tumor response of T cells. Today, we will introduce an anti-PD-1 monoclonal antibody — Toripalimab.
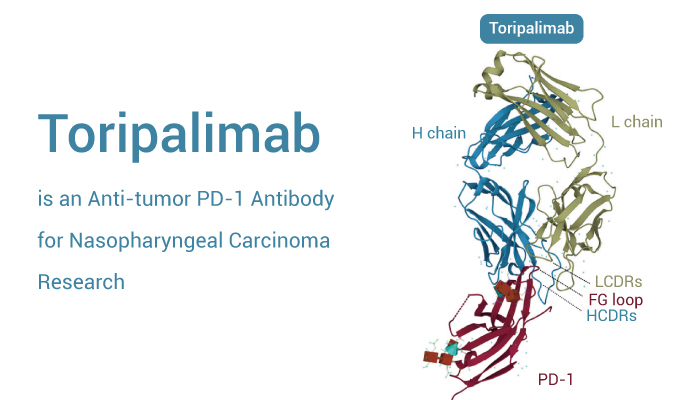
Toripalimab is a selective, recombinant, humanized monoclonal antibody against PD-1.
Toripalimab is the first domestic anti-tumor PD-1 antibody in China. It has received a conditional approval for the treatment of unresectable or metastatic melanoma that has not responded to previous systemic therapy in December, 2018. Firstly, Toripalimab is able to bind to PD-1 and block the interaction with its ligands. Secondly, the binding of Toripalimab to PD-1 is mainly attributed to the heavy chain of the former and the FG loop of the latter. Thirdly, Toripalimab is a checkpoint inhibitors with well-tolerated in the enrolled patients.
Toripalimab received a conditional approval in China for the treatment of melanoma (second-line) in December, 2018. It has also received approvals to treat nasopharyngeal carcinoma (first-line and third-line) and urothelial carcinoma (second-line) in 2021. Furthermore, the US Food and Drug Administration approve several orphan drug designations to toripalimab.
In a word, Toripalimab has exhibited primary anti-tumor effects in tumors such as melanoma, lung cancer, digestive tract tumors, hepatobiliary and pancreatic tumors, neuroendocrine neoplasms, nasopharyngeal carcinoma and urothelial carcinoma.
Reference:
[1] Zhang L, et al. Front Immunol. 2022 Jan 12;12:730666.