mTOR (mammalian target of Rapamycin) is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell motility, cell survival, protein synthesis, and transcription. mTOR belongs to the phosphatidylinositol 3-kinase-related kinase protein family. The mTOR forms two structurally and functionally distinct complexes called the mammalian target of Rapamycin complex 1 (mTORC1) and mammalian target of Rapamycin complex 2 (mTORC2).
mTORC1 integrates signals from multiple growth factors, nutrients, and energy supply to promote cell growth when energy is sufficient and catabolism when the body is hungry. mTORC1 mainly regulates cell growth and metabolism, while mTORC2 mainly controls cell proliferation and survival. It influences transcription and protein synthesis by integrating various signal stimulation, and finally regulates apoptosis, growth, and autophagy of cells. Scientists have also linked mTOR to various disease processes, such as tumor formation, arthritis, insulin resistance and osteoporosis. Among them, mTOR plays a key role in tumor tumorigenesis and development. And multiple studies have suggested that tumors typically over-activate the AKT/mTOR signaling pathway. Therefore, mTOR inhibitors has potential in the research of targeted therapy for tumors, organ transplantation, rheumatoid arthritis, and other diseases.
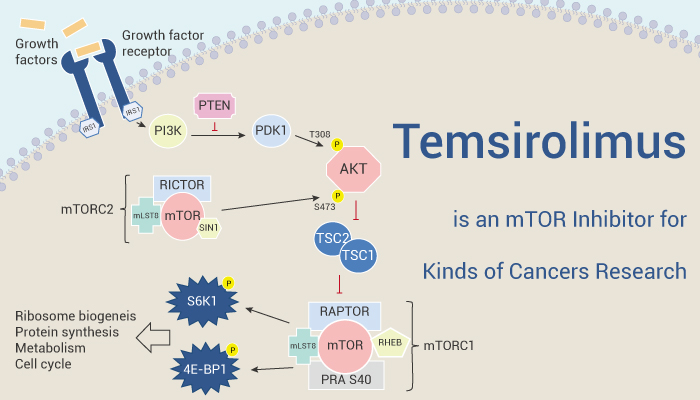
Temsirolimus (CCI-779) is an inhibitor of mammalian target of Rapamycin (mTOR) kinase
Temsirolimus binds to an abundant intracellular protein, FKBP-12, and in this way forms a complex that inhibits mTOR signaling. The disruption of mTOR signaling suppresses the production of proteins that regulate progression through the cell cycle and angiogenesis. Temsirolimus displays a modest and selective antiproliferative activity via FKBP12-dependent mechanism, but can completely inhibit the proliferation of a broad panel of tumor cells at low micromolar concentrations, involving FKBP12-independent suppression of mTOR signaling. Besides, Temsirolimus potently inhibits proliferation and induces apoptosis in primary human lymphoblastic leukemia (ALL) cells. Temsirolimus inhibits the growth of both prostate cancer xenografts. In the NOD/SCID xenograft models with human ALL, Temsirolimus treatment produces a decrease in peripheral blood blasts and in splenomegaly. What’s more, inhibition of mTOR by Temsirolimus improves performance on four different behavioral tasks and decreases aggregate formation in a mouse model of Huntington disease.
All in all, Temsirolimus is an inhibitor of mTOR that has potential in the research of cancer.
Reference:
[1] Zou Z, et, al. Cell Biosci. 2020 Mar 10:10:31.
[2] Hudes G, et, al. N Engl J Med. 2007 May 31;356(22):2271-81.