On November 16, 2023, the U.S. Food and Drug Administration has approved Repotrectinib. Repotrectinib is indicated for the treatment of adult patients with ROS1-positive locally advanced or metastatic non-small cell lung cancer (NSCLC).
ROS1 is a transmembrane receptor tyrosine kinase proto-oncogene that has been shown to have rearrangements with several genes in glioblastoma, NSCLC, and other neoplasms, including intrachromosomal fusion with GOPC due to microdeletions at 6q22.1. ROS1 fusion events are important findings in these tumors, as they are potentially targetable alterations with newer tyrosine kinase inhibitors.
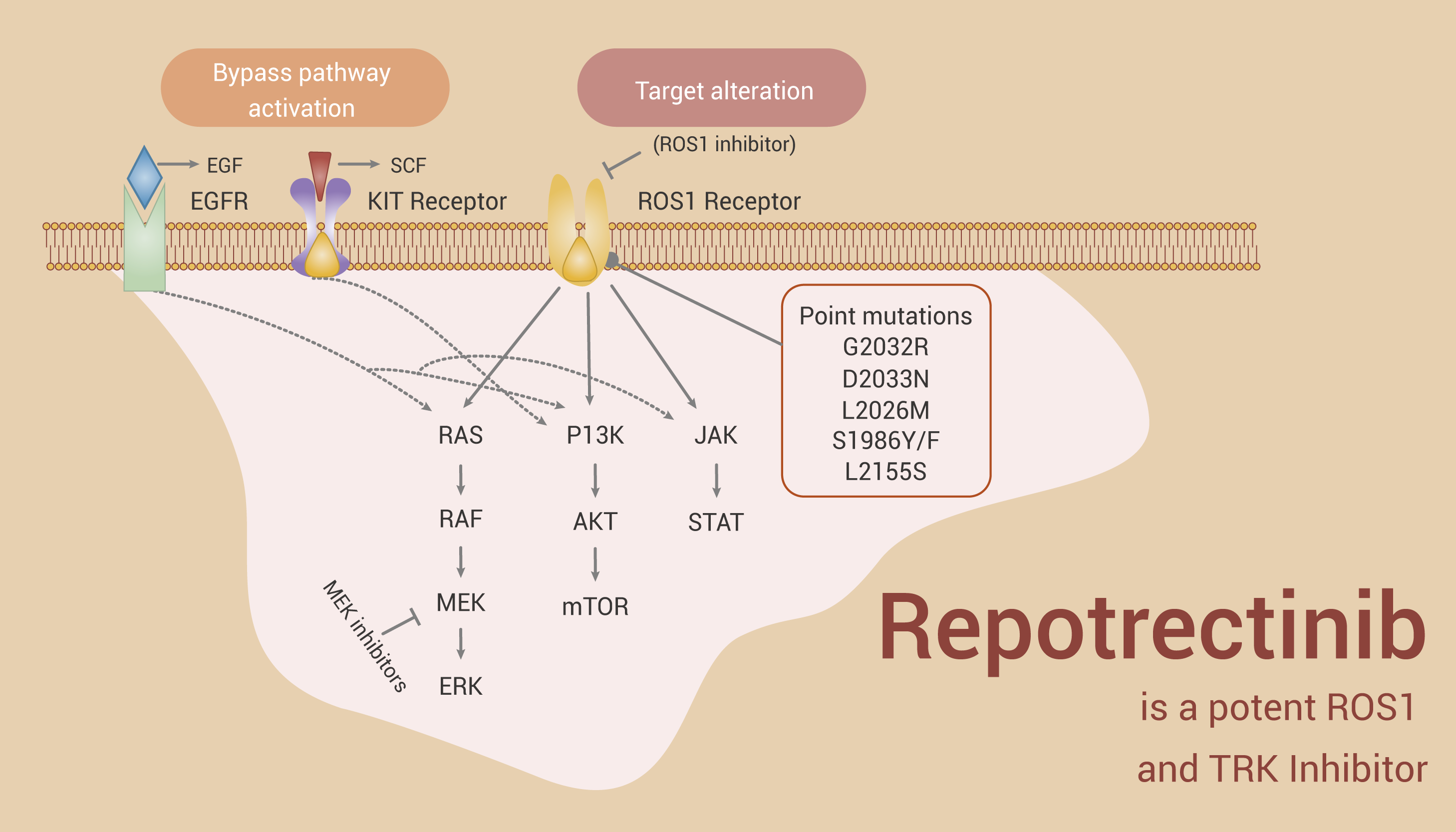
Repotrectinib is a ROS1 Inhibitor for ROS1-positive Non-small Cell Lung Cancer Research
Repotrectinib (TPX-0005) is a potent ROS1 inhibitor with an IC50 value of 0.07 nM. And it’s also a TRK inhibitor with an IC50 value of 0.83/0.05/0.1 nM for TRKA/B/C. What’s more, Repotrectinib potently inhibits WT ALK with an IC50 value of 1.01 nM. Repotrectinib has anti-cancer activity.
In Vitro, Repotrectinib inhibits mutant ALKs including ALK G1202R (IC50=1.26 nM) and ALK L1196M (IC50=1.08 nM). It also inhibits a variety of other kinases, including JAK2, LYN, Src, and FAK (IC50=1.04, 1.66, 5.3, and 6.96 nM, respectively). Repotrectinib effectively overcomes this primary resistance (IC50=100 nM in cell proliferation assay) with strong inhibition of the phosphorylation of EML4-ALK (IC50=13 nM) and the SRC substrate paxillin (IC50=107 nM). Repotrectinib inhibits H2228 cell migration in a wound healing assay with similar activity to saracatinib. In Vivo, Repotrectinib effectively inhibits tumor growth in ALK WT and ALK G1202R xenografts.
In conclusion, Repotrectinib is a ROS1 Inhibitor for ROS1-positive non-small cell lung cancer research.
Reference:
[1]. Cancer Research. July 2016.
[2]. EBioMedicine. 2018 Mar;29:112-127.