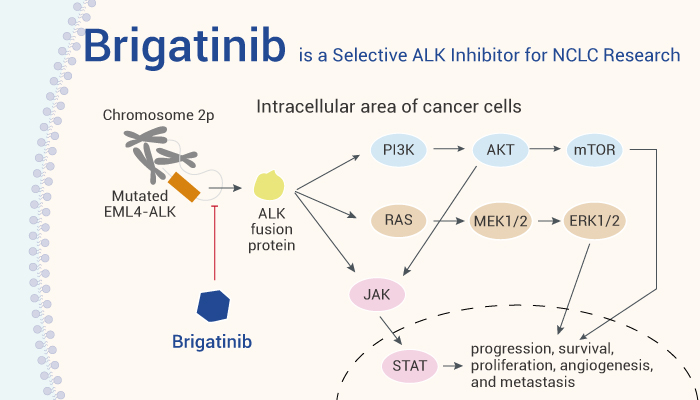
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase of insulin receptor superfamily. In addition, ALK is relevant with solid and hematologic cancers, such as none small cell lung cancers (NSCLCs). Moreover, NSCLCs with the activating gene rearrangements in ALK (ALK+) can lead to the resistance to the first-generation ALK inhibitor Crizotinib. It’s reported that more than 10 secondary mutations in ALK are associated with Crizotinib resistance in patients. Noticeably, Brigatinib, a second generation and selective ALK inhibitor. And In vitro, Brigatinib is about 12-fold more potent than Crizotinib against ALK. Brigatinib inhibits multiple clinical and preclinical Crizotinib-, Ceritinib-, and/or Alectinib-resistant ALK mutants. Furthermore, FDA approved Brigatinib for ALK-positive metastatic NSCLCs in 2020.
Brigatinib, an orally active ALK inhibitor, can be used for NSCLC research.
In vitro, Brigatinib inhibits ALK, ALK (C1156Y), ALK (F1174L), ALK (L1196M), ALK (G1202R), ALK (R1275Q) with IC50s of 0.6, 0.6, 1.4, 1.7, 4.9, 6.6 nM respectively. In addition, Brigatinib also inhibits FLT3 and ROS1 with IC50s of 2.1 and 1.9 nM respectively. And in CLB-BAR and CLB-GE (ALK addicted cell), Brigatinib (0-100 nM, 6 h) inhibits phosphorylation of ALK, ERK1/2, ERK5 and AKT dose-dependently. Apart from this, Brigatinib (0-300 nM, 72 h) also inhibits proliferation of ALK addicted neuroblastoma cell lines.
In vivo, Brigatinib (10-50 mg/kg, p.o., once daily) leads to a dose-dependent inhibition of tumor growth in ALK+ Karpas-299 (ALCL) and H2228 (NSCLC) xenograft mouse models. Besides, compared with Crizotinib-treated mice, Brigatinib markedly enhances survival of mice bearing ALK+ brain tumors. Moreover, Brigatinib also shows antitumor activity in mice bearing L1196M- and G1202R-mutant ALK.
In conclusion, Brigatinib is an orally active ALK inhibitor, and shows potent antitumor activity in various in vivo model. Therefore, Brigatinib can be used for research of NSCLC.
References:
[1] Zhang S, et al. Clin Cancer Res. 2016 Nov 15;22(22):5527-5538.
[2] Huang WS, et al. J Med Chem. 2016 May 26;59(10):4948-64.
[3] Siaw JT, et al. Oncotarget. 2016 May 17;7(20):29011-22.