On November 16, 2023, the Food and Drug Administration approved Capivasertib with Fulvestrant for adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer.
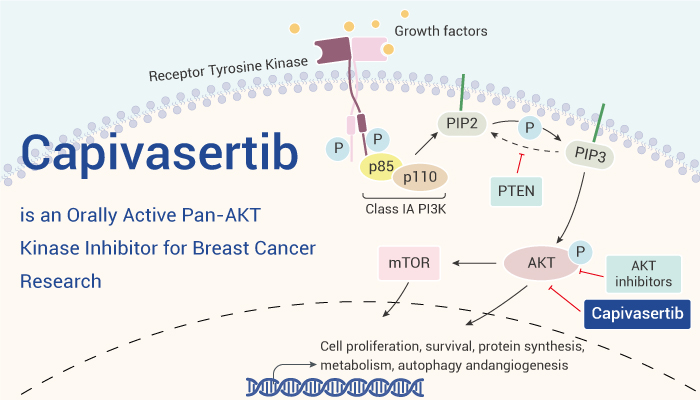
Capivasertib (AZD5363) is an orally active and potent pan-AKT kinase inhibitor with IC50 of 3, 7 and 7 nM for Akt1, Akt2 and Akt3, respectively.
In vitro: Firstly, Capivasertib (AZD5363), a novel pyrrolopyrimidine-derived compound, inhibits all AKT isoforms with a potency of 10 nM or less. Secondly, Capivasertib inhibits phosphorylation of these substrates with an IC50 value of 0.06 to 0.76 μM in the 3 cell lines. Meanwhile, Capivasertib effectively inhibits phosphorylation of S6 and 4E-BP1 in these cell lines, whereas it increases phosphorylation of AKT at both ser473 and thr308. Furthermore, in BT474c cells, Capivasertib induces FOXO3a nuclear translocation with EC50 value of 0.69 μM. A concentration of 3 μM is sufficient to almost completely localize FOXO3a to the nucleus.
In vivo: First, Capivasertib (oral) can causes dose- and time-dependent reduction of PRAS40, GSK3β, and S6 phosphorylation in BT474c xenografts. Second, Capivasertib also increases the blood glucose concentrations. And Capivasertib dose-dependent decreases the 2[18F]fluoro-2-deoxy-D-glucose (18F-FDG) uptake in U87-MG xenografts. At the same time, chronic oral dosing of Capivasertib caused dose-dependent growth inhibition of xenografts derived from various tumor types, including HER2+ breast cancer models. Finally, Capivasertib also significantly enhances the antitumor activity of RP-56976 and GW572016 in breast cancer xenografts.
To sumup, Capivasertib is an orally active pan-AKT kinase inhibitor and can be used for the research of breast cancer.
Reference:
[1] Davies BR, et al. Mol Cancer Ther. 2012 Apr;11(4):873-87.