Pyruvate kinase has four different tissue-specific isozymes in animals, PKL, PKR, PKM1, and PKM2. As reported, PKM2 is highly expressed in many types of human cancers. Besides, the dimer PKM2 regulates the rate-limiting step of glycolysis that shifts the glucose metabolism from the normal respiratory chain to lactate production in tumor cells. Therefore, PKM2 is a potential anticancer target. Meanwhile, Shikonin is a major bioactive component of a Chinese herbal medicine named zicao. Moreover, Shikonin and its derivatives have antimicrobial, antioxidant, anti-inflammatory, wound healing, anticancer, antiulcer, anti-angiogenic, and granulated tissue-forming activities.
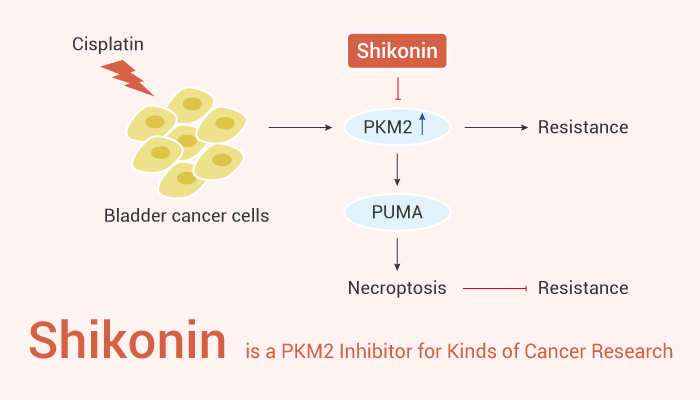
Shikonin, a PKM2 inhibitor, can be used for various cancer research.
In vitro, Shikonin (1 μM, 24 h) inhibits 12-O-tetradecanoylphorbol 13-acetate (TPA)-Induced mitochondrial malfunction and proliferation of skin epidermal JB6 cells. Besides, Shikonin (2.5-7.5 μM, 48-72 h) inhibits proliferation, migration, invasion of U87 and U251 cells. In addition, Shikoninalso inhibits activity of MMP-2/9, expression of p-β-catenin. Apart from anticancer effect, Shikonin is also an anti-inflammatory agent. Specifically, Shikonin (5 μM, 12 h) inhibits NLRP3 and AIM2 inflammasomes in iBMDMs by direct inhibiting caspase-1.
In vivo, Shikonin (10 mg/kg/day, i.p., 4 days) inhibits inflammation and chondrocyte apoptosis in a osteoarthritis rat model. Afterward, Shikonin suppresses the increases in IL-1β, TNF-α, and iNOS levels. Simultaneously, it increases caspase-3 activity and COX-2 protein expression. Furthermore, Shikonin (4 mg/kg; i.p.; daily for 7 days) prolongs the survival period of mice bearing P388 leukemia, actively suppresses proteasomal activity. Besides, Shikonin inhibits tumor growth in H22 allografts (4 or 8 mg/kg; i.p.; daily for 7 days) and PC-3 xenografts (5 mg/kg; i.p.; twice a week).
In general, Shikonin is a PKM2 inhibitor. Moreover, Shikonin shows potent anti-inflammatory and anticancer effects in vitro and in vivo.
References:
[1] Yadav S, et al. Front Pharmacol. 2022 Jul 1;13:905755.
[2] Li W, et al. Mol Carcinog. 2014 May;53(5):403-12.
[3] Zhang FY, et al. Int J Mol Sci. 2015 Oct 9;16(10):23823-48.