Sphingosine kinase (SphK) is an oncogenic lipid kinase and a key regulator of the sphingolipid metabolic pathway. The SphK isoforms (SphK1 and SphK2) catalyze the conversion of the pro-apoptotic substrate D-erythro-sphingosine (Sph) to the pro-growth/survival product sphingosine-1-phosphate (S1P). In addition, over-expression of SphK1/SphK2, have been associated with oncogenic transformation and growth in soft agar. In a cancerous setting, over-expression of the SphKs deregulates the “sphingolipid rheostat”, increasing Sphingosine-1-Phosphate (S1P) production at the expense of Ceramide/Sphingosine. Thus, S1P driven pro-mitogenic/pro-survival signaling predominates and cancer cells become dependent upon S1P signaling (“non-oncogene” addiction). Sph/Cer is important for the induction of apoptosis, and SphK over-expression also effectively depletes Sph/Cer levels in cancer cells, making them more resistant to chemotherapies. thus, inhibition of SphK may have two effects simultaneously: decreasing pro-mitogenic/pro-survival S1P signaling and increasing levels of pro-apoptotic sphingolipids (Sph/Cer). In this study, SKI-178 is a potent SphK1 and SphK2 inhibitor.
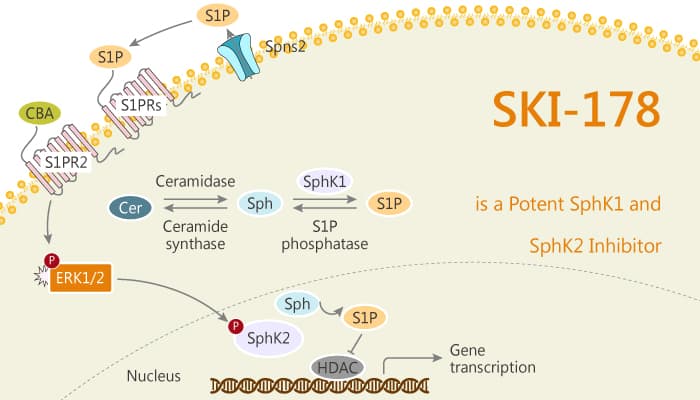
SKI-178 is a potent SphK1/SphK2 inhibitor. it directly targets engages SphK1 and SphK2 protein in cells. It also directly impairs microtubule assembly. SKI-178 is cytotoxic at IC50 concentrations ranging from 1.8 to 0.1 μM in both drug-sensitive and multi-drug resistant cancer cell lines. In addition, it induces apoptosis in a CDK1-dependent manner in human acute myeloid leukemia cell lines. Furthermore, SKI-178 induces complete remission in the MLL-AF9 mouse model system. Meanwhile, it results in a dose-dependent increase in survival.
In summary, SKI-178 is an SphK1/2 inhibitor. It is cytotoxic to a broad range of cancer cell lines including multi-drug resistant types. SKI-178 is safe and effective in mouse models of AML, supporting its further development as a multi-targeted anti-cancer therapeutic agent.
Reference:
Hengst JA, et al. Cancer Transl Med. 2017;3(4):109-121.