Protein arginine methyltransferases (PRMTs) play a crucial role in a variety of biological processes. PRMTs divide into three categories. Firstly, type I PRMTs catalyzes mono- and asymmetric dimethylation of arginine residues. Secondly, type II PRMTs catalyzes mono- and symmetric dimethylation of arginine residues. Finally, type III PRMT catalyzes only monomethylation of arginine residues. Consequently, overexpression of PRMTs implicates in various human diseases including cancer.
In this study, Mohammad S. Eram, et al report the discovery of MS023. Especially, MS023 is a potent, selective and cell-active inhibitor of human type I PRMTs. Importantly, MS023 displays high potency for type I PRMTs including PRMT1, 3, 4, 6 and 8, but is completely inactive against type II and type III PRMTs, protein lysine methyltransferases and DNA methyltransferases. Moreover, MS023 potently decreases cellular levels of histone arginine asymmetric dimethylation. Researchers assesse the effect of pharmacological inhibition of endogenous PRMT1 by MS023 in cells. As a result, MS023 treatment (48 h exposure) potently and concentration-dependently reduces cellular levels of H4R3me2a (IC50=9 nM). MS023 (20 h exposure) concentration-dependently reduces the H3R2me2a mark in HEK293 cells (IC50=56 nM).
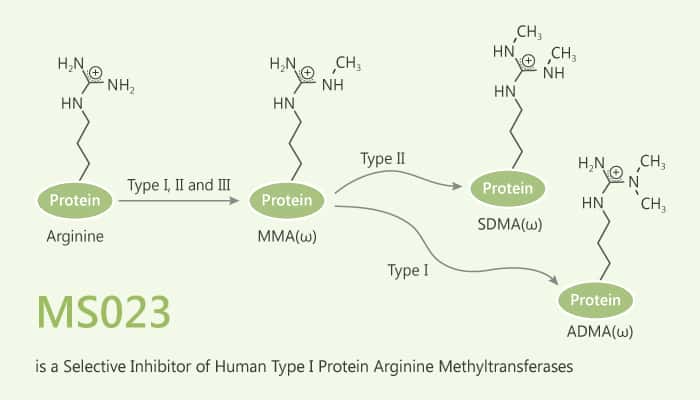
All in all, MS023 is a potent, selective and cell-active inhibitor of type I PRMTs. In biochemical assays, MS023 is highly potent and selective for type I PRMTs over a broad range of epigenetic modifiers including type II and type III PRMTs, PKMTs, DNMTs, histone lysine demethylases, and methyl-lysine and methylarginine reader proteins. Importantly, MS023 potently inhibits the catalytic activity of PRMT1 and reduced levels of H4R3me2a in cells.