Breast cancer is the most frequently diagnosed cancer of women worldwide. The feature of triple-negative breast cancers (TNBCs) is the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 receptor (HER2/ERBB2) overexpression.
Although early-stage TNBC responds well to treatment, the advanced disease is worse overall survival when compares with other breast cancer subtypes.
Cyclin-dependent kinases (CDKs) are serine/threonine kinases the activity of which depends on the interaction with a cyclin regulatory subunit.
The CDK family of enzymes orchestrate the regulation of key eukaryotic cellular processes. It includes the cell cycle and transcription. CDKs regulate transcription via phosphorylation of the C-terminal domain (CTD) of RNA polymerase II (RNA Pol II).
SR-4835 is a potent dual inhibitor of CDK12/CDK13 kinase. This article will briefly clarify its activity in vitro and in vivo.
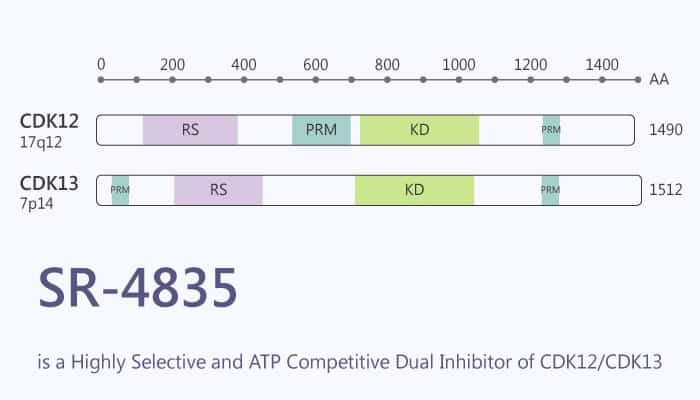
First of all, in a molecular modeling study, the X-ray structure of CDK12 predicts that SR-4835 is ATP competitive. It interacts via hydrogen bonding with the hinge region of the kinase (Tyr-815, Met-816, and Asp-819). In a kinase assay, SR-4835 is highly selective toward CDK12 and CDK13 among 450 kinases at 10 μM in the assay. It inhibits less than 20% activity when a test against full-length active LRRK2 (at 10 μM).
Nextly, In-Cell Western assays, SR-4835 blocks Ser2 phosphorylation with an EC50 value of 100 nM. Although BRD4 inhibition links with reduced-phosphorylation of the CTD region at Ser2.
SR-4835 has no affinity to BRD4 at any of the concentrations, nor does it inhibit PARP activity.
In MDA-MB-231 cells, SR-4835 can reduce the expression of a cast of DDR genes due to its inhibition of CDK12/13 kinase activity.
Ultimately, researchers apply an orthotopic patient-derived xenograft (PDX) model (PDX4013). There are four groups exist in the test: vehicle, SR-4835, cisplatin, or the combination of SR-4835 and cisplatin. SR-4835 or cisplatin results in an enormous decrease in tumor growth, where 20% of the mice show complete tumor regression in mice.
Meanwhile, the combination treatment provokes rapid tumor regression without toxicity. It is striking even more where 50% of this cohort lacked detectable disease.
In summary, SR-4835, as an orally bioavailable inhibitor of CDK12/13, it has excellent isoform selectivity and few off-target interactions. Additionally, SR-4835 augments the anticancer activity of cisplatin, irinotecan, and olaparib. Moreover, it is well tolerated in mice after long-term dosing.
SR-4835 is promising for breast cancer therapy, and the use of optimized SR-4835 analogs in the oncology clinic is in prospect.
reference:
Quereda V, et al. Cancer Cell. 2019 Nov 11;36(5):545-558.e7.