The aldehyde dehydrogenase (ALDH) superfamily has 19 protein-coding family members with varying tissue or development expression. In addition, ALDHs metabolize endogenous and exogenous aldehydes to their corresponding carboxylic acids to prevent the accumulation of toxic aldehydes. Besides, ALDH1A enzymes are also involved in the biosynthesis of retinoic acid (RA). Moreover, ALDHs mediate resistance to chemotherapy via direct drug metabolism and by regulation of reactive oxygen species. Other studies have used knockdown of select ALDH1 family members to support their role in chemotherapy resistance. Furthermore, ALDH activity is essential to chemotherapy resistance in breast cancer, and ALDH1A2 and ALDH1A3 are implicated in chemotherapy resistance in mesothelioma. Given the role of ALDH1A isozymes in chemotherapy resistance, stem cell biology, and its link with CSC, ALDH1A activity has been as a therapeutic target in cancer. CM10 is a potent and selective aldehyde dehydrogenase 1A family inhibitor (ALDH1Ai).
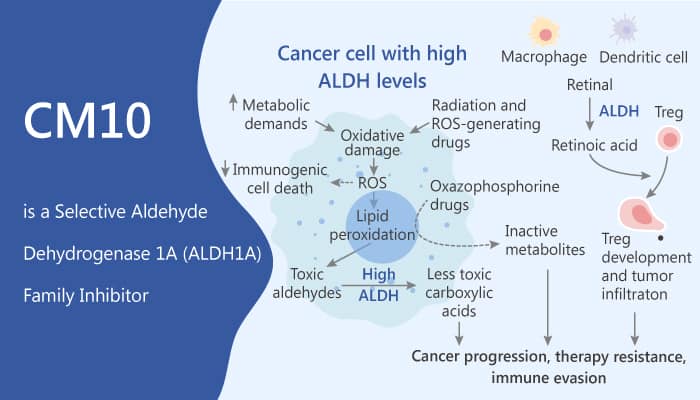
CM10 is a potent and selective aldehyde dehydrogenase 1A family inhibitor (ALDH1Ai), with IC50s of 1700, 740, and 640 nM for ALDH1A1, ALDH1A2, and ALDH1A3, respectively. Specifically, CM10 does not inhibit any of the other ALDH family members. Meanwhile, CM10 can regulate metabolism and has anti-cancer activity. Similar to 673A, CM10 inhibits ALDEFLUOR activity in live cells, and preferentially depletes CD133+ cells. Nonetheless, PubChem searches suggested CM10 and 673A (active in 17 of 673 and 11 of 556 assays, respectively) had limited and non-overlapping, potential off-target activity. Together, this suggests that CD133 cell depletion is an on-target effect of the ALDH1Ai. All in all, CM10 is a potent and selective aldehyde dehydrogenase 1A family inhibitor and regulates metabolism and has anti-cancer activity.
References:
Chefetz I, et al. Cell Rep. 2019 Mar 12;26(11):3061-3075.e6.