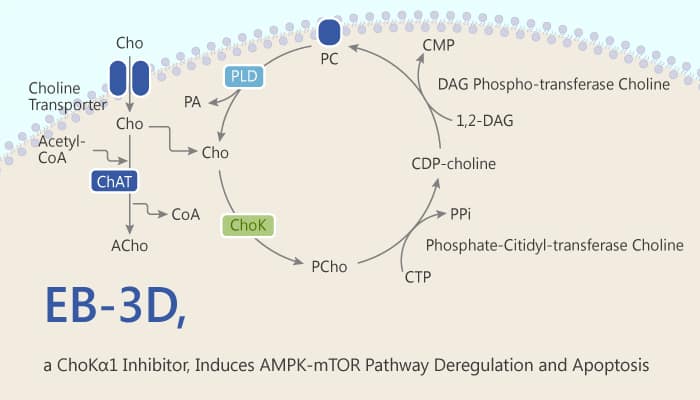
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematologic disorder. It results from the malignant transformation of T-cell progenitors. Since the overexpression of choline kinase alpha 1 (ChoKa1) has been associated with tumorigenesis in many cancers, it becomes an interesting therapeutic target. ChoK catalyzes the phosphorylation of choline (Cho) to phosphocholine (PCho) in the first step of the CDP-choline branch of Kennedy’s pathway for phosphatidylcholine (PC) synthesis. In humans, there are three isoforms of ChoK: ChoKα1, ChoKα2, and ChoKβ. It proposes ChoKα1 isoform as an oncogenic promoting factor. The overexpression and overactivation of ChoKα lead to abnormal choline metabolism, resulting in higher levels of phosphocholine (PCho) and in general, total choline-containing compounds (tCho). In this context, ChoKα is a potential therapeutic target. EB-3D is a potent and selective choline kinase alpha 1 (ChoKα1) inhibitor. It induces deregulation of the AMPK-mTOR pathway and apoptosis in leukemia T-cells.
EB-3D displays excellent antiproliferative activity against a wide cohort of T-leukemic cell lines, with GI50 values in the nanomolar range and resulting in more cytotoxic. Moreover, the most sensitive cell line is MOLT-16 with GI50 of 0.9 ± 0.6 nM. In addition, EB-3D induces a dose-dependent increase in apoptotic cells in T-ALL cell lines after 72 h of treatment. EB-3D enhances the chemotherapeutic effects of these drugs, significantly lowering their GI50. In particular, the most prominent effect is l-asparaginase. Furthermore, MEK1/2 S271/S221 and ERK1/2 T202/Y204 phosphorylated after EB-3D treatment in healthy lymphocytes. However, at the present stage, how EB-3D targets the AMPK-mTOR pathway remains unknown.
In summary, choline kinase inhibition could be a valuable therapeutic strategy in T-ALL treatment. EB-3D may be an interesting new molecule worthy of further evaluation as a potential chemotherapeutic agent.
Reference:
Mariotto E, et al. Biochem Pharmacol. 2018 Sep;155:213-223.