Topoisomerases are proteins that cut the DNA backbone. Especially, topoisomerases let the cut ends twist past each other to a more relaxed configuration. Moreover, topoisomerases reseal the phosphodiester bonds in the backbone to again form an intact double helix. DNA topoisomerases I and II present in cells acts through scission of the DNA backbone on one or two strands, respectively. In this study, researchers designed and synthesized topoisomerase I inhibitor TP3011. TP3011 is the active metabolite of TP3076. In addition, TP3011 and TP3076 are equipotent to SN38 in terms of topoisomerase 1 inhibition.
DNA topoisomerases bind covalently to the DNA phosphate. DNA topoisomerases break the phosphodiester linkage between neighboring nucleotides, storing the energy in that bond to use in the process of resealing the bond. Furthermore, DNA topoisomerases unravel twists in DNA that occur as a result of DNA transcription and replication.
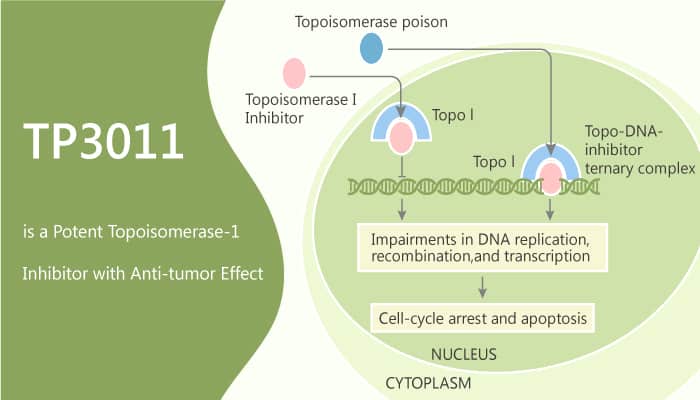
Topoisomerase I is a ubiquitous enzyme whose function in vivo is to relieve the torsional strain in DNA. Topoisomerase I specifically removes positive supercoils generated in front of the replication fork and to relieve negative supercoils occurring downstream of RNA polymerase during transcription. TP300 is converted non-enzymatically to TP3076, then metabolized to TP3011 by aldehyde oxidase 1 (AOX1). TP3011 and TP3076 are equipotent as Topo-1 inhibitors, with IC50 in the sub-nanomolar range HCT-116 colorectal cancer cells in vitro. TP3011 shows antiproliferative activities against human cancer cell lines with IC50s of 0.85, 8.5, and 8.2 nM for colorectal cancer HCT116, non-small cell lung carcinoma QG56, and NCI-H460, respectively.
In summary, TP3011 is a topoisomerase I inhibitor with improved anti-tumor activities.