Heat shock protein 70 (HSP70) are chaperone proteins that protect proteins from stress and promote nascent polypeptide folding. They exist in both prokaryotic and eukaryotic organisms. The stress-inducible HSP70 is an emerging cancer target that directly inhibits apoptosis. Administration of the inhibitors for HSP90 induces the expression of HSP70. In particular, HSP90 itself is an extremely important cancer target. Therefore, targeting HSP70 will result in a dual knockdown not only disrupting its anti-apoptotic function but also impairing its function as critical HSP90 cochaperone. The eukaryotic HSP70 family includes HSP70, HSC70, and HSPA5 (also known as Bip, Grp78). HM03 is a HSPA5 inhibitor.
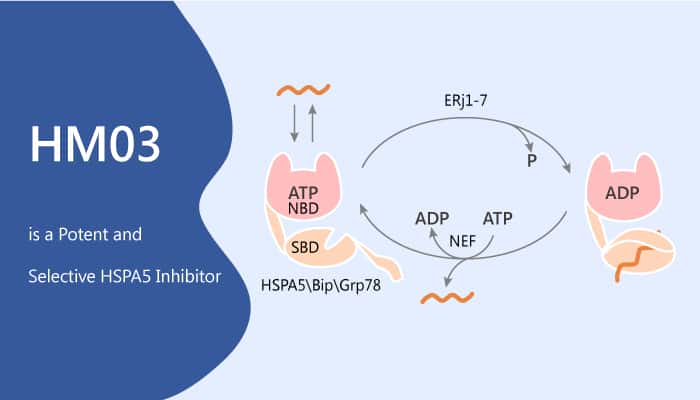
HSPA5 is a stress response protein
HSPA5, is part of the HSP70 family and plays a role in the folding and assembly of proteins in the endoplasmic reticulum. Agents or conditions that adversely affect endoplasmic reticulum function may induce HSPA5. HSPA5 plays a key role in monitoring protein transport through the cell. HSPA5 is crucial for the proper glycosylation, folding as well as for the maintenance of cell homeostasis and the prevention of apoptosis. Furthermore, HSPA5 guides posttranslational hepatitis B virus large envelope protein import into the mammalian endoplasmic reticulum. HSPA5 actively regulates multiple malignant phenotypes, including cell growth, migration, and invasion.
In this study, researchers discovered the lead compound of HSP70 HM03. HM03 demonstrates activities in the primary assay and exhibits prominent inhibition effect (18% survival at 25 μM). Moreover, HM03 becomes cytotoxic when concentrations are higher than the effective concentrations. HM03 exhibits over 50% inhibition at 25 μM concentration in tumor cell based assay. In addition, HM03 displays promising selectivity towards the substrate binding channel of HSPA5. To summarise, HM03 is a potent and selective HSPA5 inhibitor with anticancer activity.