Protein kinases are enzymes that catalyze protein phosphorylation. Obviously, Protein kinases include many types of kinases, such as PKC,PKA, c-Fgr and phosphorylase kinase. Thereinto, PKC, PKA and phosphorylase kinase belong to serine or threonine kinases and c-Fgr belongs to Tyr kinases.
Interestingly, diacylglycerol (DG) can activate PKC and the second messenger cyclic adenosine can activate PKA. Specifically, PKC structure includes four conserved regions C1-C4, and the C3 region includes an ATP-binding sequence Gly-X-Gly-X-X-Gly-Lys. Furthermore, C3 has high homology with the ATP-binding sites of other protein kinases. Moreover, the function of protein kinase A is to transfer phosphate groups on ATP to serine or threonine residues of specific proteins for phosphorylation. Thus, proteins phosphorylated by protein kinase can regulate the activity of target proteins.
Here, we focus on the protein kinases inhibitor, Staurosporine.
Staurosporine is an ATP-competitive inhibitor of protein kinases. Notably, Staurosporine is a potent, ATP-competitive and non-selective inhibitor of protein kinases with IC50s of 6, 15, 2 and 3 nM for PKC, PKA, c-Fgr, and phosphorylase kinase, respectively. Alternatively, Staurosporine is an apoptosis inducer.
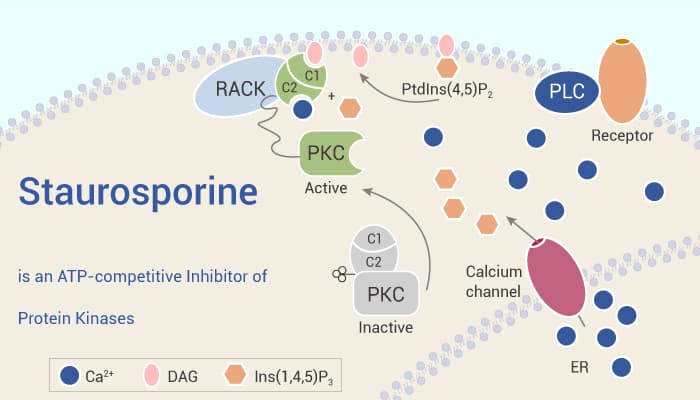
In vitro, Staurosporine, as a protein kinase C (PKC) inhibitor with a broad spectrum of activity, is an alkaloid isolated from the culture broth of Streptomyces staurospores. In vivo, Staurosponne attenuates the impaired perlormance of water maze and passive avoidance tasks, even though the drug administration began 2 weeks after the lesion. Moreover, Staurosporine slightly inhibits tumor promotion of Teleocidin, even at the dose at which Staurosporine itself induced tumors.
Taken together, Staurosporine is an ATP-competitive protein kinases inhibitor. To sum up, Staurosporine is a potent compound of cancer research.
References:
[1]. Meggio F, et al. Eur J Biochem. 1995 Nov;234(1):317-322.