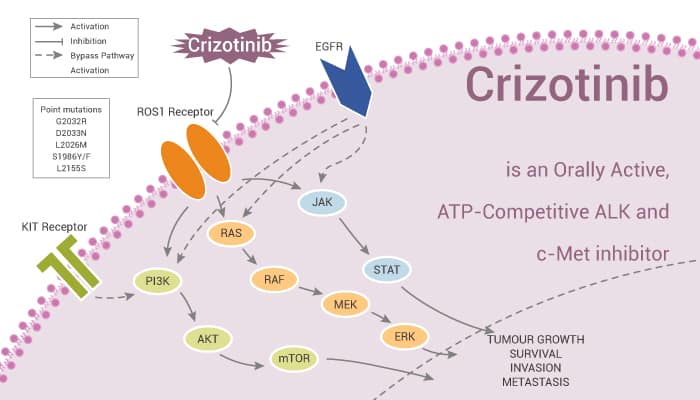
In this article, we will introduce an orally bioavailable, ATP-competitive ALK and c-Met inhibitor, Crizotinib.
The IC50 values for ALK and c-Met kinases are 20 and 8 nM, respectively. Additionally, in cell-based assays, Crizotinib inhibits tyrosine phosphorylation of NPM-ALK and tyrosine phosphorylation of c-Met with IC50s of 24 and 11 nM, respectively.
In mIMCD3 mouse or MDCK canine epithelial cells, Crizotinib displays similar potency against c-Met phosphorylation. The IC50 values are 5 nM and 20 nM, respectively. Additionally, Crizotinib shows improved or similar activity against NIH3T3 cells engineered to express c-Met ATP-binding site mutants V1092I or H1094R or the P-loop mutant M1250T. The IC50 values are 19 nM, 2 nM and 15 nM, respectively. While the IC50 value in NIH3T3 cells expressing wild-type receptors is 13 nM.
Crizotinib also potently prevents the phosphorylation of c-Met in NCI-H69 and HOP92 cells, with IC50 of 13 nM and 16 nM, respectively. Which express the endogenous c-Met variants R988C and T1010I, respectively[1].
Crizotinib also potently inhibits NPM-ALK phosphorylation in Karpas299 with an IC50 of 24 nM. What’s more, Crizotinib potently prevents cell proliferation, which is associated with G(1)-S-phase cell cycle arrest. It induces apoptosis in ALK-positive ALCL cells with an IC50 of 30 nM, but not ALK-negative lymphoma cells.
Crizotinib exhibits inhibitory effects in tumor growth.
In the GTL-16 model, Crizotinib reveals the ability to cause marked regression of large established tumors (>600 mm3) in both the 50 mg/kg/day and 75 mg/kg/day treatment. Additionally, It leads to a 60% decrease in mean tumor volume over the 43-day administration schedule.
Besides, in another study, Crizotinib displays the ability to completely inhibit GTL-16 tumor growth for >3 months. And only 1 of 12 mice exhibits a significant increase in tumor growth over the 3-month treatment schedule at 50 mg/kg/day.
What’s more, Crizotinib leads to a significant dose-dependent reduction of CD31-positive endothelial cells in GTL-16 tumors. And in the GTL-16 and U87MG models, Crizotinib displays a significant dose-dependent reduction of human VEGFA and IL-8 plasma levels. Notably, Crizotinib results in a marked inhibition of phosphorylated c-Met, Akt, Erk, PLCλ1, and STAT5 levels in GTL-16 tumors.
In summary, Crizotinib is a potent ALK, and c-Met inhibitor and exhibits anti-cancer activities.
[1]. Zou HY, et al. Cancer Res. 2007, 67(9), 4408-4417.
[2]. Christensen JG, et al. Mol Cancer Ther. 2007, 6(12 Pt 1), 3314-3322.
[3]. Cui JJ, et al. J Med Chem. 2011 Sep 22;54(18):6342-63.