Bcl-2 protein family is a key regulator of apoptosis and a marker of B-cell lymphoma. Importantly, apoptosis is an important physiological process of selective cell clearance, involving a variety of biological events. Particularly, the Bcl-2 family is the most characteristic protein family involved in regulating apoptotic cell death. It is composed of anti-apoptotic and pro-apoptotic members. Obviously, the Bcl-2 family has three groups, including pro-apoptotic promoters, pro-apoptotic effectors, and anti-apoptotic proteins. Members of the Bcl-2 protein family share sequence homology within a conserved region called the Bcl-2 homology (BH) domain, which determines structure and function.
In the survival-promoting subfamily, Mcl-1, Bcl-xL (Bcl-2l1), Bcl-2a1, and Bcl-b, like Bcl-2, inhibit the initiation of apoptosis. Interestingly, Bcl-2 is the first gene to promote prolonged cell survival rather than increased proliferation. It is highly expressed in many hematological malignancies to varying degrees, providing protection from carcinogenesis and cell death induced by external pressure. Additionally, BH3 mimetics include a new class of Bcl-2 inhibitors. Whether used as a single drug or in combination with other anticancer drugs, they show promising results in several hematological malignancies. Here, we will introduce a BH3 mimetic, ABT-737.
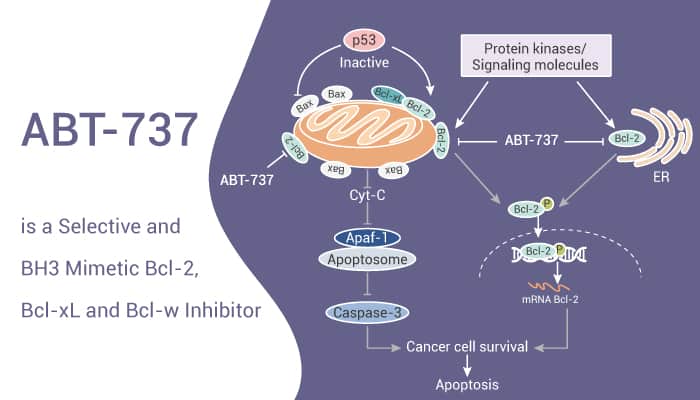
ABT-737 is a Selective and BH3 Mimetic Bcl-2, Bcl-xL and Bcl-w Inhibitor.
At first, ABT-737, a BH3 mimetic, has EC50s of 30.3 nM, 78.7 nM, and 197.8 nM for Bcl-2, Bcl-xL and Bcl-w, respectively. Specifically, ABT-737 induces the disruption of the BCL-2/BAX complex and BAK-dependent but BIM-independent activation of the intrinsic apoptotic pathway. Besides, ABT-737 induces autophagy and has the potential for acute myeloid leukemia (AML) research.
Secondly, ABT-737 binds BCL-2, BCL-XL, and BCL-W with high affinity (Ki<1 nM). But it binds weakly (Ki>460 nM) to other antiapoptotic BCL-2 family members, including MCL-1 and BFL-1. Moreover, ABT-737 binds the BH3-binding groove of BCL-XL and BCL-2. ABT-737 with 100 nM for 1-72 hours induces apoptosis and synergizes with chemotherapy in HL-60 cells. Furthermore, ABT-737 causes approximately 80% of HCT116 cell death. The BAX knockout variant is completely resistant to ABT-737. ABT-737 has no effect on cell cycle distribution.
Thirdly, ABT-737 disrupts BCL-2/BAX heterodimerization and induces BAX conformational change in HL-60 leukemic cells. Meanwhile, ABT-737 induces a BAX/BAK-dependent impairment of maximal O2 consumption rate in sensitive cells. Stable BCL-2 overexpression in MCF10A cells induces an ABT-737-sensitive primed for death state. Nonetheless, ABT-737 induces dose-dependent impairment of maximal O2 consumption rate in B-cell lymphoma cells.
Finally, ABT-737 with 20, 30 mg/kg/day by i.p. for 21 days suppresses the leukemia burden by 48% and 53%, respectively, in human leukemia xenograft. In fact, ABT-737 significantly extends the survival of mice in this aggressive leukemia model.
References:
Konopleva M, et al. Cancer Cell. 2006 Nov;10(5):375-88.