Autophagy refers to the natural and conservative degradation of cells through lysosome dependent regulation mechanism. Specifically, it allows the orderly degradation and recovery of cellular components. At present, there are four forms of autophagy: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and coprophagy. The catabolic process of macroautophagy is evolutionarily conserved in all eukaryotic cells. It includes five steps: initiation, nucleation, extension, fusion, and degradation. Besides, autophagy is mediated by a unique organelle called an autophagosome. Moreover, autophagy is generally a non-selective degradation system because autophagosomes engulf part of the cytoplasm.
Furthermore, autophagy is an intracellular degradation system that transports cytoplasmic components to lysosomes. Autophagy is a self-degradation process. Meanwhile, this is very important for balancing the energy source in the critical period of development and coping with nutritional stress. Autophagy plays a housekeeping role in removing misfolded or aggregated proteins, removing damaged organelles, eliminating intracellular pathogens. Nonetheless, autophagy defects are relevant to a variety of human diseases, including neurodegenerative diseases and cancer. Now, we will introduce a specific and potent autophagy inhibitor, Spautin-1.
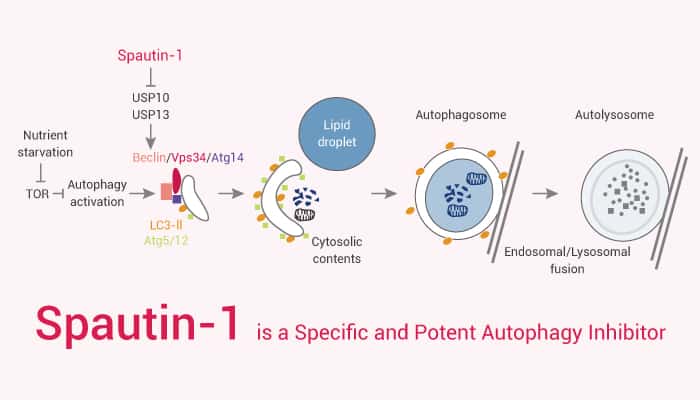
Spautin-1 is a Specific and Potent Autophagy Inhibitor.
First of all, Spautin-1 inhibits ubiquitin-specific peptidases, USP10 and USP13 with IC50s of 0.6-0.7 μM. Interestingly, Spautin-1 enhances imatinib mesylate (IM)-induced CML cell apoptosis by reducing the expression of the anti-apoptotic proteins Mcl-1 and Bcl-2. The pro-apoptotic activity of Spautin-1 has related to the activation of GSK3β, an important downstream effector of PI3K/AKT. Importantly, Spautin-1 enhances IM-induced cytotoxicity in CML cell line K562, decreasing the IC50 from 1 to 0.5 μM. The mechanism of spautin-1 acting on acute pancreatitis is relevant to impaired autophagy inhibition.
In the second place, Spautin-1 ameliorates the pathogenesis of acute pancreatitis induced by cerulein or L-arginine. Particularly, Spautin-1 pretreatment significantly diminishes the elevation of serum amylase and lipase levels, which are indicative of trypsin activity. Spautin-1 inhibits the increasing levels of serum TNFα caused by Cerulein. Obviously, Spautin-1 treatment can ameliorate the inflammation damage induced by cerulein, such as edema, degeneration, coagulative necrosis, and infiltration of inflammatory cells.
All in all, Spautin-1 is a specific and potent autophagy inhibitor.
References:
Shao S, et al. Int J Oncol. 2014 May;44(5):1661-1668.