The protein tyrosine phosphatase SHP2 (also called SH-PTP2, Syp, PTP1D, and PT-P2C) is a ubiquitously expressed 68 kDa non-receptor PTP. In the inactive state, the SH2 domains of SHP2 auto-inhibit the catalytic PTP domain by binding intramolecularly to the defined acidic residues. Upon activation, several receptor tyrosine kinases, including MET, EGF, and PDGF, recruit adaptor proteins such as GAB and GRAB to form a protein complex. In addition, the gene encoding SHP2, PTPN11, is an oncogene in the development of several types of leukemia and solid tumors. Somatic mutations in PTPN11 account for about 35% of juvenile myelomonocytic leukemia (JMML) patients. Several types of leukemia including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and acute lymphoid leukemia (ALL) found somatic gain-of-function mutations. In addition, PTPN11 mutations occur in solid tumors, including lung adenocarcinoma and colon cancer. In this study, GS-493 is a selective protein tyrosine phosphatase SHP2 inhibitor.
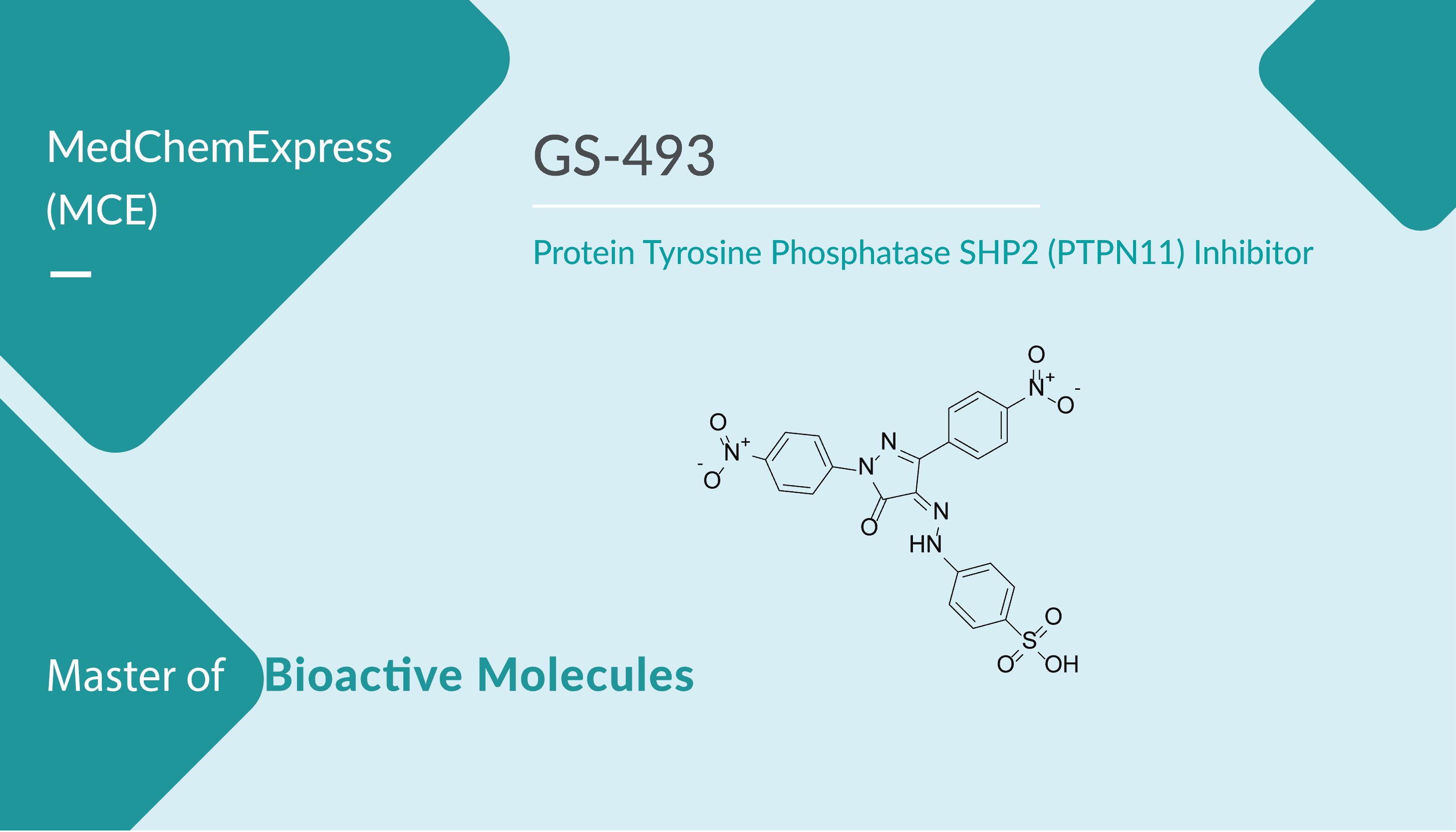
GS-493 is a selective SHP2 inhibitor with an IC50 of 71 nM. It is 29- and 45-fold more active toward SHP2 than related SHP1 and PTP1B. GS-493 blocks cellular motility and the growth of cancer cells. GS-493 blocks HGF-stimulated epithelial-mesenchymal transition of human pancreatic adenocarcinoma (HPAF) cells. It also blocks the growth of the human NSCL cancer cell line LXFA 526L in soft agar. Moreover, decreases the number of tumor cell colonies to 32%. In addition, GS-493 inhibits tumor growth significantly in a murine xenograft model.
In summary, SHP2 is a relevant protein target for the inhibition of mobility and invasiveness of cancer cells. GS-493 blocks cellular motility and growth of cancer cells in vitro and in vivo.
Reference:
Grosskopf S, et al. ChemMedChem. 2015;10(5):815-826.