Actinomycin D is isolated from Streptomyces species, containing a phenoxazine chromophore and two cyclic pentapeptide lactone rings. Besides, Actinomycin D can inhibit DNA repair, DNA-directed RNA synthesis, or DNA synthesis via binding to double-stranded DNA and the phenoxazine chromophore inserting DNA bases. Therefore, it acts as a DNA intercalator and as a minor groove binding agent. Since the 1950s, Actinomycin D had been found to have anticancer activity and applied to clinical practice. This drug is widely used in researching various cancer, such as sarcomas, germ cell cancers, melanoma, and so on.
According to research, Actinomycin D stimulates DNA topoisomerase I-induced cleavage at specific DNA sites and irritates DNA cleavage through DNA topoisomerase II.
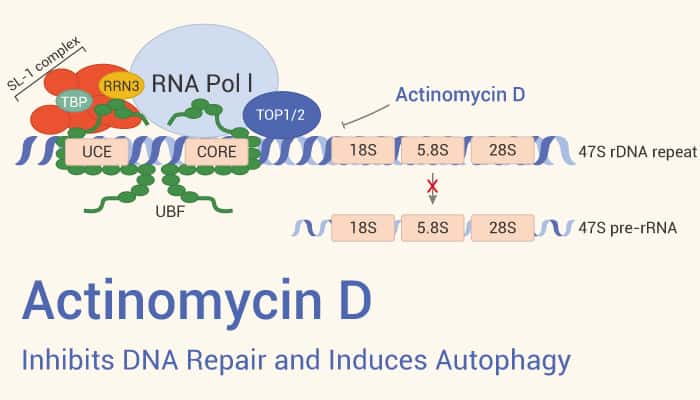
Because the guanine base has a preferred orientation with respect to the chromophore of Actinomycin D. All deoxyribodinucleotides containing guanine will form a complex with this drug.
In vitro and in vivo Studies
In cell experiments, Actinomycin D can markedly reduce the proliferation of vascular smooth muscle cells. It arrests cell cycle at G1-phase, and exhibits an IC50 of 0.4 nM and lethal dose LD50 of 260 μM. Besides, it can cause induction of apoptosis in primary chronic lymphocytic leukemia cells and B-cell lymphoma cell lines in a p53-independent manner. The experiment results show that Actinomycin D suppresses mRNAs expression of BCL2, LYN, and TOSO which are important for CLL cell survival.
In vivo studies, Actinomycin D substantially reduces the thickness of neointima in rats when applied topically surrounding the carotid adventitia. In the TCL1-mouse model, the drug (0.06 mg/kg; IV for 10 days) reduces tumor load, and also strongly suppresses BCL2 protein levels that blocks apoptosis, in the neoplastic compartment.
In conclusion, Actinomycin D is a potent anticancer agent, inhibiting DNA repair, and DNA synthesis, and inducing cancer cells apoptosis.
Reference
[1] Richard B. Silverman, et al. ISBN 9780123820303, 2014, 275-331.
[2] Wu CH, et al. J Biomed Sci. 2005;12(3):503-512.
[3] Merkel O, et al. Leukemia. 2012;26(12):2508-2516.