Oncolytic peptides offer yet a new therapeutic modality. Their membranolytic mode of action includes the release of danger-associated molecular pattern molecules (DAMPs) and tumor antigens from cancer cells. Therefore, it results in regression of solid tumors and systemic tumor-specific immune responses. Consequently, the development of oncolytic peptides into novel anticancer therapeutics has emerged as a promising immunotherapeutic strategy.
LTX-315 (KKWWKKW-Dip-K-NH2) is an oncolytic peptide with potent anticancer activity; inhibits MRC-5, A20, and AT84 with IC50s of 34.3, 8.3, and 11 μM, respectively.
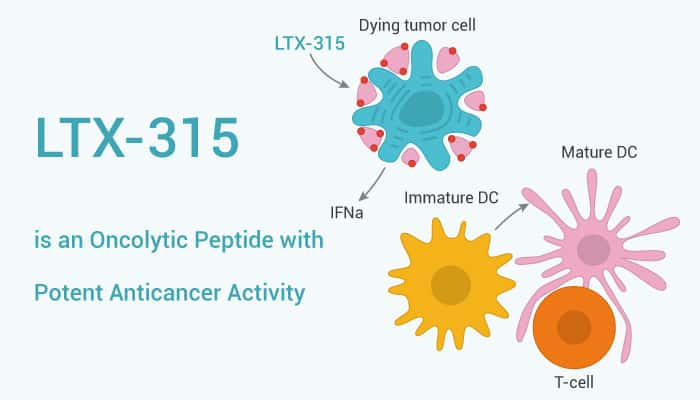 LTX-315 is equipotent against drug-resistant cancer cells and nontoxic towards red blood cells. It also shows high plasma protein binding and degrades rapidly to non-toxic metabolites. LTX-315 also induces the rapid killing of cancer cells. The oncolytic activity of LTX-315 stems from both a direct lytic effect on the plasma membrane in addition to permeabilization of the mitochondrial membrane, leading to cellular death by necrosis and the release of tumor antigens. Treatment of cancer cells with LTX-315 causes the release of several danger signals (DAMPs) that are associated with immunogenic cell death and stimulation of adaptive immune responses.
LTX-315 is equipotent against drug-resistant cancer cells and nontoxic towards red blood cells. It also shows high plasma protein binding and degrades rapidly to non-toxic metabolites. LTX-315 also induces the rapid killing of cancer cells. The oncolytic activity of LTX-315 stems from both a direct lytic effect on the plasma membrane in addition to permeabilization of the mitochondrial membrane, leading to cellular death by necrosis and the release of tumor antigens. Treatment of cancer cells with LTX-315 causes the release of several danger signals (DAMPs) that are associated with immunogenic cell death and stimulation of adaptive immune responses.
Intratumoral administration of LTX-315 has resulted in complete regression and systemic tumor-specific immune responses in several preclinical models. Intratumoral administration of LTX-315 also results in tumor necrosis and the infiltration of immune cells into the tumor parenchyma followed by complete regression of the tumor in the majority of the animals. Moreover, LTX-315 induces the release of danger-associated molecular pattern molecules such as the high mobility group box-1 protein in vitro and the subsequent upregulation of proinflammatory cytokines such as interleukin (IL) 1β, IL6, and IL18 in vivo. Animals cured by LTX-315 treatment are protected against a re-challenge with live B16 tumor cells both intradermally and intravenously.
Reference:
[1].Haug BE, et al. J Med Chem. 2016 Apr 14;59(7):2918-27.
[2].Camilio KA, et al. 2014 Jun;63(6):601-13.