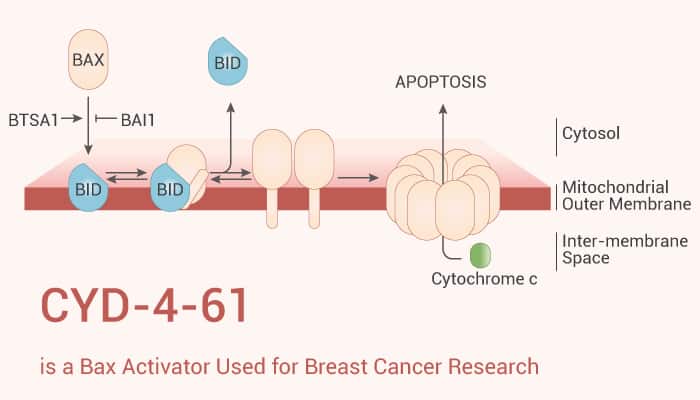
Bax is an apoptosis regulator, belonging to the Bcl-2-like protein family. It controls mitochondrial dysfunction and apoptosis. Bax can promote apoptosis by forming a heterodimer with Bcl-2 to become a lethal mitochondrial pore protein. Specifically, it can induce mitochondrial outer membrane permeability, release cytochrome c, and cause programmed death of cancerous cells. This feature makes Bax a hot executioner protein in inhibiting cancer progression.
Nowadays, the prevalence of breast cancer remains high, which is the main type of female cancer. Drug resistance in breast cancer treatment is mainly due to cancer cells escaping drug-induced apoptosis. More importantly, Bax is significantly downregulated in breast cancer cells, so increasing the expression of Bax can restore the drug sensitivity of malignant cells. Here, we will introduce an effective Bax activator, CYD-4-61.
CYD-4-61 is a potent Bax activator to inhibit multiple breast cancer types.
In vitro experiments, CYD-4-61 (0-10 μM; 72 h) shows significantly superior anti-breast cancer proliferation activity. The IC50 of MDA-MB-231 and ER-positive MCF-7 cell lines for triple-negative breast cancer is 0.07 µ M and 0.06 µ M, respectively. CYD-4-61 (5 μM and 10 μM; 24 h) also increases the cell number with apoptotic bodies in breast cancer cells, indicating an increased apoptosis rate. CYD-4-61 (5 μ M; 0-48 h) decreases the level of p-Bax but increases the levels of total Bax protein, cytochrome c, and apoptosis-related proteins (PARP-1, caspase 3).
Furthermore, CYD-4-61 can translate in vitro anticancer activity into in vivo antitumor efficacy. It (2.5 mg/kg; i.p.; once daily for 7 d) inhibits triple-negative breast tumor growth in mouse xenograft models.
All in all, CYD-4-61 activates Bax, consequently inducing mitochondrial outer membrane penetration, and cytochrome c release, finally resulting in cancer cell apoptosis.
Reference:
[1] Liu G, et al. Eur J Med Chem. 2019 Sep 15;178:589-605.