CD123, the alpha chain of the interleukin 3 receptor (IL-3Rα), is a molecule found on cells that helps transmit the signal for interleukin 3, a soluble cytokine important to the immune system. Besides, Interleukin 3 (IL-3) is a soluble and pleiotropic cytokine. Additionally, IL-3 regulates the function and production of hematopoietic and immune cells. IL-3 binds to ligand-specific α subunits, resulting in heterodimerization with common/shared signaling β subunits. CD123 acts as a cytokine receptor overexpressed in various blood lymphocytic neoplasms. And blood lymphocytic neoplasms include acute myeloid leukemia, blastic plasmacytoid dendritic cell neoplasms, acute lymphoblastic leukemia, hairy cell leukemia, and systemic mast cell polycythemia.
Furthermore, CD123 is a useful diagnostic and therapeutic biomarker in hematological malignancies, and efforts to target CD123 have led to the development of drugs that effectively target tumor cells expressing this molecule.
Pivekimab sunirine (IMGN 632) is a CD123-targeting ADC for hematological malignancies.
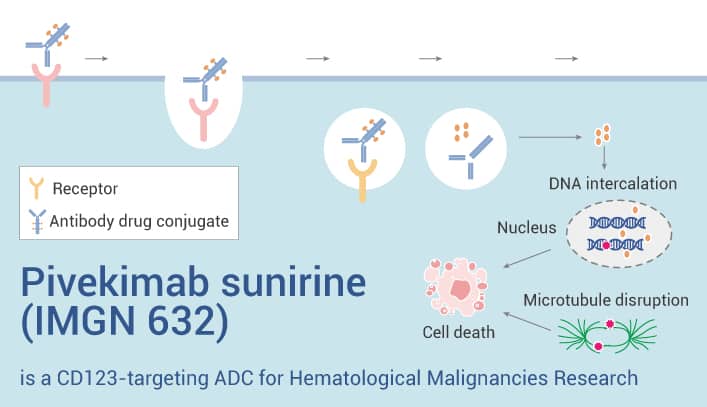
Pivekimab sunirine (IMGN 632) is an antibody-drug conjugate (ADC) targeting CD123. It comprises a high-affinity CD123 antibody, a cleavable linker, and an indolinobenzodiazepine pseudodimer (IGN) payload. Pivekimab sunirine can be used for research on hematological malignancies. In some in vitro studies, Pivekimab sunirine(0-1 nM, 4-6 days) exhibits cytotoxicity in AML cell lines such as EOL-1, HNT-34, KASUMI-3, KG-1, KO52, MOLM-13, MV4-11, SHI-1, SKM-1, UCSD-AML1 cells with the IC50 ranges from 0.6 pM to 40 pM. Additionally, In a mouse xenograft model of human AML, Pivekimab sunirine (40-240 μg/kg, i.p.) caused tumor regression and induced complete and durable tumor regression at 240 μg/kg. Besides, Pivekimab sunirine (8 and 80 μg/kg, i.p.) prolongs lifespan in mice with disseminated Molm-13 xenograft.
In conclusion, Pivekimab sunirine (IMGN 632) is a CD123-targeting antibody-drug conjugate (ADC) for the study of hematologic malignancies.
References:
[1] Naval Daver, et al. Blood (2022) 140 (Supplement 1): 145-149.
[2] Yelena Kovtun, et al. Blood Adv. 2018 Apr 24;2(8):848-858.