Alzheimer’s disease (AD) is one of the most common neurodegenerative diseases, accounting for more than 80% of dementia cases worldwide. AD leads to the progressive loss of mental capacity and behavior, with a functional decline in the ability to learn. HDAC6 belongs to the HDAC family, and its structure contains a cytoplasmic anchoring domain, which mediates its stable anchorage in the cytoplasm. The enzymatic activity of HDAC6 is exerted on tubulin, Hsp90, and cortactin substrates; thus it is a key regulator of the cytoskeleton and cell migration. HDAC6 interacts with tau in human brain tissues, and the inhibition of HDAC6 attenuates tau phosphorylation at T231, a critical regulatory site for tau function; however, it does not disrupt the HDAC6–tau interaction. Furthermore, HDAC6 inhibition also appeared to downregulate Aβ aggregation and improve cognition in an AD mouse model. MPT0G211 is a potent, orally active and selective HDAC6 inhibitor.
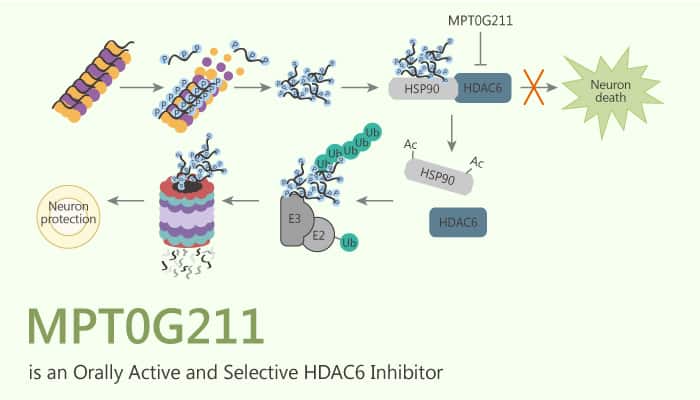
MPT0G211, an orally active and selective HDAC6 inhibitor (IC50=0.291 nM), ameliorates tau phosphorylation, and cognitive deficits in an Alzheimer’s disease model.
It displays >1000-fold selective for HDAC6 over other HDAC isoforms. Moreover, it can penetrate the blood-brain barrier. Furthermore, it significantly inhibits the phosphorylation of tau Ser396. MPT0G211 inhibits HDAC6/Hsp90 binding and causes subsequent proteasomal degradation of polyubiquitinated proteins. In addition, MPT0G211 significantly decreases the phosphorylation of tau by GSK3β inactivation. MPT0G211 also significantly attenuates the phosphorylation of tau Ser396 and Ser404 in both cell lines. MPT0G211 inhibits MDA-MB-231 and MCF-7 cells growth.
In AML cells, MPT0G211 potentiated the cytotoxic effects of DOXO by impairing DNA repair machinery and activating Bcl-2-associated X protein (BCL-XL)-dependent cell apoptosis. It also significantly ameliorates the spatial memory impairment. MPT0G211 not only diminishes tau phosphorylation by inhibition GSK3β activity but also enhances the acetylation of Hsp90, which causes the downregulation of HDAC6/Hsp90 binding and facilitates proteasomal degradation of polyubiquitinated p-tau.
In summary, MPT0G211 appears to be a promising strategy for improving the AD phenotypes, including tau hyperphosphorylation and aggregation, neurodegeneration, and learning and memory impairment, making it a valuable agent for further investigation.
Reference:
Fan SJ, Huang FI, et al. Cell Death Dis. 2018;9(6):655. Published 2018 May 29.