Pancreatic cancer is cancer that’s found anywhere in the pancreas. Histone deacetylases (HDACs) regulate the post-translational modification of histones and consequent gene expression. HDACs act as transcriptional repressors and overexpress in several types of cancer, including pancreatic cancer. HDAC inhibitors are effective in killing pancreatic cancer cells. In this study, researchers developed an inhibitor of HDACs AES-135.
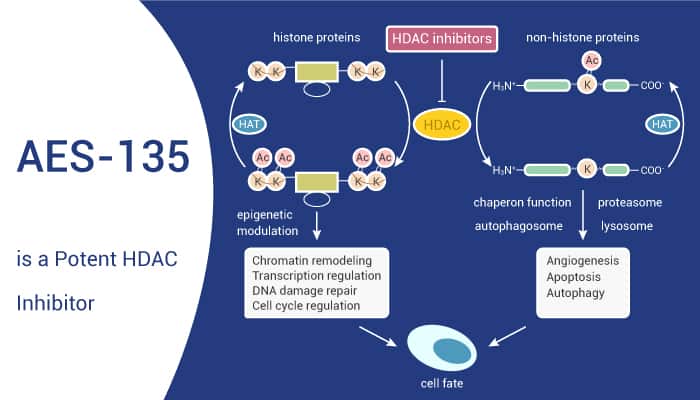
AES-135 exhibits nanomolar inhibitory activity against HDAC3, HDAC6, and HDAC11 in biochemical assays. Especially, AES-135 biochemically inhibits HDACs 3, 6, 8, and 11 with IC50 values of 190–1100 nM. Moreover, AES-135 prolongs survival significantly in an orthotopic murine model of pancreatic cancer. AES-135 exhibits selective in vitro cytotoxicity in low-passage patient-derived pancreatic cancer cells even in the presence of cancer-associated fibroblast (CAF) cells. Mice treated with AES-135 show significantly increased survival, with a median survival rate of 36.5 days compared to 29.5 days for the vehicle mice. AES-135 has other favorable in vivo properties such as metabolic stability in mouse hepatocytes and bioavailability in micromolar (μM) concentrations in NSG mice for >10 h (intraperitoneal (IP) injection).
To effectively treat pancreatic cancer, single-agent therapy has not been effective; therefore, individualized combinations of targeted therapies would be necessary for making therapeutic advances in this devastating disease. Combination studies including AES-135 in animal models are important in order to determine whether the addition of HDAC inhibitors to standard-of-care agents or a new combination, such as immune checkpoint inhibitors, would dramatically extend survival.
All in all, AES-135 is an HDAC inhibitor that prolongs survival in an orthotopic mouse model of pancreatic cancer.