Src homology 2 domain-containing phosphatase (SHP2) is a phosphatase that mediates signaling downstream of multiple receptor tyrosine kinases. SHP2 suppresses signaling through the MAPK pathway. Furthermore, SHP2 inhibition has demonstrated tumor growth inhibition in receptor tyrosine kinases-activated cancers.
In this study, researchers report the discovery of IACS-13909. IACS-13909 is a specific and potent allosteric inhibitor of SHP2. Moreover, IACS-13909 potently impedes the proliferation of tumors harboring a broad spectrum of activated receptor tyrosine kinases as the oncogenic driver. IACS-13909 potently suppresses the phosphatase activity of purified full-length, recombinant human SHP2 protein with an IC50 of approximately 15.7 nM. The Kd of IACS-13909 binding to SHP2 is approximately 32 nM. IACS-13909 is highly selective for SHP2. When tested at 10 μM against a panel of 22 phosphatases, IACS-13909 only shows significant inhibition of SHP2.
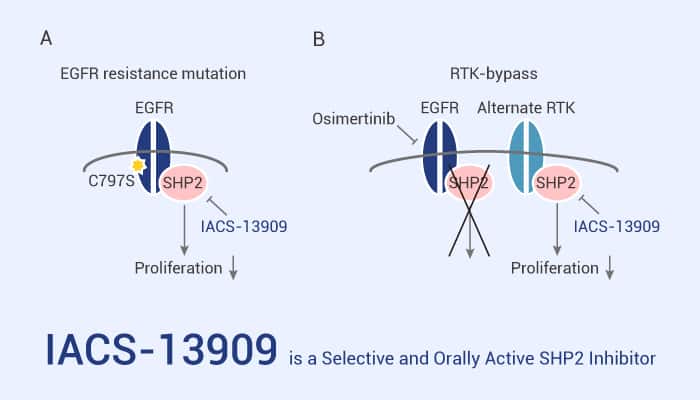
IACS-13909 inhibits the proliferation and MAPK pathway signaling of tumor cell lines driven by a broad spectrum of receptor tyrosine kinases in vitro. In particular, IACS-13909 inhibits the proliferation and MAPK pathway signaling in receptor tyrosine kinases-activated cancer cells in vitro due to on-target SHP2 inhibition. IACS-13909 (70 mg/kg; for 21 days) potently suppresses tumor growth, with 100% tumor growth inhibition (TGI) in mice. Especially, the treatment was well-tolerated, with bodyweight maintained throughout the study. Importantly, IACS-13909 exhibits antitumor efficacy in Osimertinib-resistant models that harbor clinically relevant resistance mechanisms. Besides, the combination of IACS-13909 and Osimertinib extends the durability of Osimertinib response in Osimertinib-sensitive tumors.
All in all, ACS-13909 is a potent and selective allosteric SHP2 inhibitor. In addition, IACS-13909 has antitumor activity and suppresses the MAPK pathway.