The KRAS oncoprotein is a GTPase and an essential mediator of intracellular signaling pathways. KRAS oncoprotein regulates tumor cell growth and survival. However, KRAS also is the most frequently mutated oncogene in cancer and encodes a key signaling protein in tumors. In addition, KRASG12C mutant is present in approximately 13% of lung adenocarcinoma (including NSCLC), 3% of colorectal cancer (CRC), and 2% of other solid tumors. Here, we will introduce the first KRAS (G12C) inhibitor in clinical development-Sotorasib (AMG-510).
Sotorasib is an orally active KRASG12C covalent inhibitor.
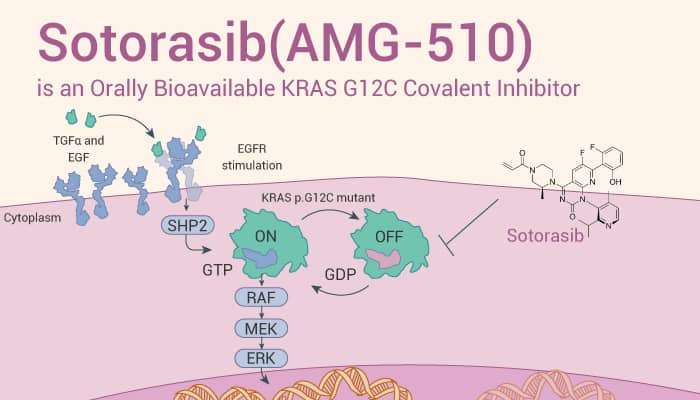
In cellular assays, Sotorasib (AMG-510) covalently modifies KRAS G12C and inhibits KRAS G12C signaling as measured by phosphorylation of ERK1/2 (p-ERK) in all KRAS p.G12C-mutant cells. In addition, Sotorasib (1-10 μM; 72 hours) also potently impairs cellular viability in both NCI-H358 and MIA PaCa-2, with IC50≈0.006 μM and 0.009 μM, respectively. And, Non-KRAS (G12C) lines are insensitive to Sotorasib (IC50>7.5 μM). Furthermore, selective inhibition of KRAS(G12C) by Sotorasib results in increased T-cell infiltration and activation.
In preclinical tumor models, Sotorasib rapidly and irreversibly binds to KRAS (G12C). Then, Sotorasib provides durable suppression of the mitogen-activated protein kinase (MAPK) signaling pathway. Moreover, Sotorasib (orally; once daily) is capable of inducing tumor regression in mouse models of KRAS (G12C) cancer.
In immune-competent mice, treatment with Sotorasib results in a pro-inflammatory tumor microenvironment and produces durable cures alone as well as in combination with immune-checkpoint inhibitors. Moreover, cured mice reject the growth of isogenic KRAS (G12D) tumors, which suggests adaptive immunity against shared antigens.
All in all, as a first-in-class oral KRAS (G12C) inhibitor, Sotorasib is hopeful to be an effective anti-tumor agent. Furthermore, in clinical trials, Sotorasib demonstrates anti-tumor activity in the first dosing cohorts and represents a potentially transformative therapy for patients for whom effective treatments are lacking.
Reference:
- Canon J, et al. Nature. 2019 Nov;575(7781):217-223.
- Karen Rex, et al. Cancer Res (2019) 79 (13_Supplement): 3090.