Acute myeloid leukemia (AML) is a group of malignant diseases of the hematopoietic system. And, AML occurs as the result of mutations in hematopoietic stem/progenitor cells. Despite advances in treatment, AML remains a challenging disease to manage, with a high relapse rate and poor long-term outcomes. In this article, we will introduce a potent potent and ATP-competitive FLT3/AXL inhibitor, Gilteritinib. Gilteritinib is providing new hope for patients with AML.
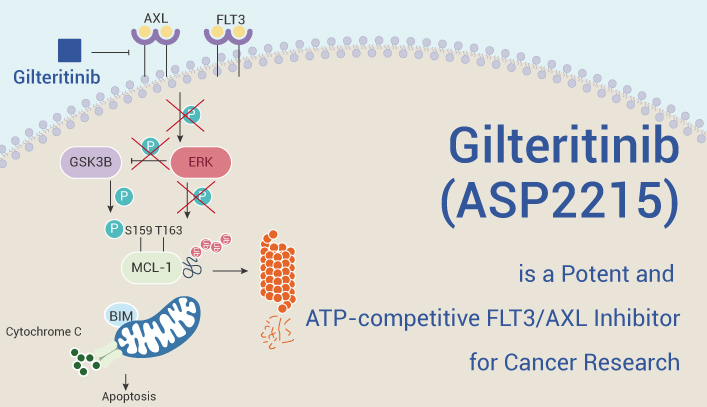
Gilteritinib (ASP2215) is a potent and ATP-competitive FLT3/AXL inhibitor with IC50s of 0.29 nM/0.73 nM, respectively. In a 78-kinase test, Gilteritinib inhibits FLT3 at an IC50 that is approximately 800-fold more potent than the concentration required to inhibit c-KIT (230 nM).
Preclinical studies have shown that Gilteritinib is highly effective in reducing growth and survival.
Gilteritinib has antiproliferative effects against MV4-11 and MOLM-13 cells. Gilteritinib inhibits the growth of MV4-11 and MOLM-13 cells with mean IC50s of 0.92 nM (95% CI: 0.23-3.6 nM) and 2.9 nM (95% CI: 1.4-5.8 nM), respectively. What’s more, Gilteritinib inhibits FLT3 phosphorylation by 57%, 8%, and 1% with 0.1 nM, 1 nM, and 10 nM Gilteritinib, respectively.
In MV4-11 xenografted mice, Gilteritinib for 28 days results in dose-dependent inhibition of MV4-11 tumor growth. Additionally, it induces complete tumor regression at more than 6 mg/kg. Further, Gilteritinib decreases tumor burden in the bone marrow and prolonged the survival of mice intravenously transplanted with MV4-11 cells.
Moreover, One of the key highlights of Gilteritinib is its ability to target a specific mutation that is common in AML, making it a promising therapeutic option for patients with FLT3-mutated AML. Furthermore, Gilteritinib has shown synergistic effects with chemotherapy drugs commonly used in the treatment of AML, such as Cytarabine and Daunorubicin.
In conclusion, Gilteritinib represents a major advance for AML research. While further research is needed to fully understand its mechanisms of action and long-term effects.
Reference:
[1] ASP2215. 2014 ASCO Annual Meeting.
[2] Mori M, et al. Invest in New Drugs. 2017 Oct;35(5):556-565.