Microtubules act as “railroads” that drive intracellular transport in cells. Specifically, they interact with auxiliary proteins to assemble into larger structures and provide a framework for the rest of the cell. Besides, microtubules are formed by α- and β-tubulin heterodimers, which assemble into long hollow polymers. Moreover, their dynamic instability allows microtubules to explore the cellular space and reshape themselves based on intracellular and extracellular signals. Microtubules are present in all characterized eukaryotic organisms. Furthermore, microtubules and their associated proteins form the mitotic spindle, a dynamic self-organizing machine that separates chromosomes during mitosis, which is crucial in all eukaryotic cell processes. Dynamic microtubules remain one of the most successful targets for cancer chemotherapy. Meanwhile, many new drugs targeting microtubules are being tested in clinical trials, and numerous microtubule-active compounds are under development. Nonetheless, the most successful microtubule-targeting chemotherapeutic drugs are Paclitaxel (Taxol) and Vinblastine. Here, we will introduce an orally bioavailable microtubule inhibitor, Ixabepilone.
Ixabepilone is an Orally Bioavailable Microtubule Inhibitor for Cancer Research.
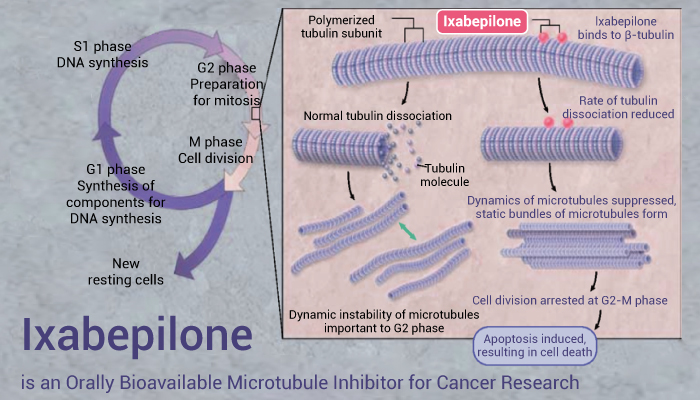
Firstly, Ixabepilone (BMS-247550) binds to tubulin and promotes tubulin polymerization and microtubule stabilization. Thereby Ixabepilone arrests cells in the G2-M phase of the cell cycle and induces tumor cell apoptosis.
Again, BMS-247550 blocks cells in the mitotic phase of the cell division cycle. Importantly, BMS-247550 shows rapid systemic clearance and extensive tissue distribution, with a terminal half-life of ∼3 h by i.v. bolus in mice. In particular, BMS-247550 demonstrates antitumor activity that is superior to paclitaxel in both paclitaxel-resistant and -sensitive tumors.
Finally, Ixabepilone is an orally bioavailable microtubule inhibitor and induces tumor cell apoptosis for cancer research.
References:
John T. Mol Cancer Ther February 2009 8; 275.